Exogenous adenosine counteracts tigecycline resistance in tet(X3)-harboring Escherichia coli
- PMID: 40622216
- PMCID: PMC12323663
- DOI: 10.1128/spectrum.02382-24
Exogenous adenosine counteracts tigecycline resistance in tet(X3)-harboring Escherichia coli
Abstract
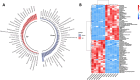
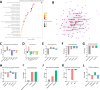
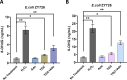
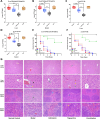
The rapid spread of antibiotic resistance poses a global health crisis. Tigecycline is a last-resort antibiotic, but the recent emergence of the plasmid-borne tet(X3) gene conferring high-level tigecycline resistance is deeply concerning. Here, we report a metabolomics-guided approach to overcome tet(X3)-mediated resistance. Using untargeted metabolomics, we identified adenosine as a key metabolic biomarker associated with tet(X3) expression. Remarkably, supplementation with exogenous adenosine was able to restore tigecycline susceptibility in tet(X3)-positive Escherichia coli both in vitro and in vivo. Our mechanistic investigations reveal that adenosine enhances the bactericidal effects of tigecycline by inducing oxidative stress, DNA/RNA damage, and cell membrane disruption in resistant bacteria. This study establishes a powerful metabolomics-driven strategy to potentiate antibiotic efficacy against drug-resistant pathogens. The adenosine-based adjuvant therapy represents a promising approach to combat the global crisis of antibiotic resistance.IMPORTANCEThe emergence and widespread dissemination of the high-level tigecycline resistance gene tet(X3) have posed a significant challenge to the efficacy of tigecycline, which serves as the "last line of defense" against antimicrobial-resistant bacteria. Although tigecycline has not been approved for veterinary clinical use, constant detection of tet(X3) genes and new subtypes in livestock farming environments poses a substantial threat to public health safety. While developing novel antibiotics is an effective approach to eradicate resistance genes/bacteria, it entails considerable costs and a lengthy timeframe. This study discovered that exogenous adenosine can effectively restore the sensitivity of tet(X3)-positive Escherichia coli to tigecycline through metabolic reprogramming based on a non-targeted metabolomics strategy. The findings are highly significant for exploring comprehensive mechanisms underlying bacterial multidrug resistance, utilizing metabolic reprogramming strategies to curb the spread of novel resistant genes, and treating clinical infections caused by tet(X3)-positive bacteria.
Keywords: adenosine; antimicrobial resistance; metabolomics; tet(X3); tigecycline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Genomic insights into tigecycline non-susceptibility in Clostridioides difficile: the role of the Tet P determinant and efflux mechanisms.BMC Microbiol. 2025 Jul 7;25(1):421. doi: 10.1186/s12866-025-04143-9. BMC Microbiol. 2025. PMID: 40624625 Free PMC article.
-
A strain defined as a novel species in the Acinetobacter genus co-harboring chromosomal associated tet(X3) and plasmid associated bla NDM-1 from a beef cattle farm in Hebei, China.Front Cell Infect Microbiol. 2025 Jul 2;15:1594982. doi: 10.3389/fcimb.2025.1594982. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40673007 Free PMC article.
-
Emergence of a conjugative hybrid plasmid co-harboring mcr-1.1, mcr-3.39, and tet(X4) in Escherichia coli from wastewater in Seoul: A potential threat to last-line antibiotics.Sci Total Environ. 2025 Sep 10;994:180039. doi: 10.1016/j.scitotenv.2025.180039. Epub 2025 Jul 8. Sci Total Environ. 2025. PMID: 40633397
-
Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev. 2017 Feb 9;2(2):CD003543. doi: 10.1002/14651858.CD003543.pub4. Cochrane Database Syst Rev. 2017. PMID: 28178770 Free PMC article.
-
Point-of-care tests for urinary tract infections to reduce antimicrobial resistance: a systematic review and conceptual economic model.Health Technol Assess. 2024 Nov;28(77):1-109. doi: 10.3310/PTMV8524. Health Technol Assess. 2024. PMID: 39644102 Free PMC article.
References
-
- Goff DA, Kullar R, Goldstein EJC, Gilchrist M, Nathwani D, Cheng AC, Cairns KA, Escandón-Vargas K, Villegas MV, Brink A, van den Bergh D, Mendelson M. 2017. A global call from five countries to collaborate in antibiotic stewardship: united we succeed, divided we might fail. Lancet Infect Dis 17:e56–e63. doi: 10.1016/S1473-3099(16)30386-3 - DOI - PubMed
-
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, Burden of AMR Collaborative Group . 2019. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19:56–66. doi: 10.1016/S1473-3099(18)30605-4 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

