Antitumor Activity of Tumor-Infiltrating Neutrophils Revealed by a Syngeneic Mouse Model of Cholangiocarcinoma
- PMID: 40622290
- PMCID: PMC12400046
- DOI: 10.1111/cas.70129
Antitumor Activity of Tumor-Infiltrating Neutrophils Revealed by a Syngeneic Mouse Model of Cholangiocarcinoma
Abstract
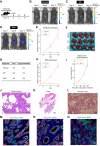
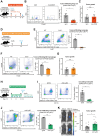
The tumor immune microenvironment plays a key role in the regulation of cancer progression. Recent studies have suggested a relation between diverse tumor genotypes and tumor immune microenvironment phenotypes for cholangiocarcinoma (CCA). However, the contribution of tumor-infiltrating immune cells to CCA progression has remained unclear, underscoring the need for genetically defined CCA models in immunocompetent mice. We here aimed to generate genetically engineered and transplantable CCA organoids from C57BL/6 mice and to investigate the role of tumor-infiltrating immune cells in CCA progression with this model. CCA organoids were generated ex vivo with the use of the CRISPR/Cas9 system. Orthotopic transplantation of CCA organoids harboring mutations in Smad4, Trp53, and Kras into wild-type C57BL/6 mice resulted in tumor formation accompanied by distant metastasis. Selective depletion of immune cell types in the tumor-bearing mice revealed an antitumor action of tumor-infiltrating neutrophils (TINs) that was mediated by direct killing of cancer cells through the production of reactive oxygen species. Furthermore, administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) increased the number and cytotoxicity of TINs, suppressed tumor growth, and prolonged the survival of tumor-bearing mice. Finally, combination treatment with rhG-CSF and standard chemotherapy resulted in a synergistic attenuation of tumor growth. Our study therefore provides a syngeneic and genetically defined mouse model of CCA and highlights the therapeutic potential of targeting TINs with rhG-CSF.
Keywords: cholangiocarcinoma; granulocyte colony‐stimulating factor (G‐CSF); neutrophil; organoid; tumor microenvironment.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Keiichi I. Nakayama is an editorial board member of
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

