Radiation dosimetry and fasting-dependent hepatobiliary clearance of the VAChT-specific PET radioligand 18F-VAT in humans
- PMID: 40622650
- PMCID: PMC12234429
- DOI: 10.1186/s13550-025-01273-z
Radiation dosimetry and fasting-dependent hepatobiliary clearance of the VAChT-specific PET radioligand 18F-VAT in humans
Abstract
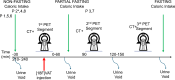
Background: The vesicular acetylcholine transporter ligand (-)-(1-((2R,3R)-8-(2-[(18)F]fluoro-ethoxy)-3-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)piperidin-4-yl)(4-fluorophenyl)-methanone (18F -VAT) enables positron emission tomography PET quantification of cholinergic dysfunction in neurologic and psychiatric disorders. Determining its bio-distribution and dose exposure in humans is essential for clinical implementation, particularly given hepatobiliary clearance observed in pre-clinical models. Based on pre-clinical data, eight healthy subjects (4 males, 4 females) received 385-533 MBq 18F-VAT immediately followed by three sequential whole-body PET/CT scans. PET data were collected under three different fasting conditions relative to administration of Ensure®Plus oral supplement and PET image acquisition: (1) complete fasting (n = 3), (2) oral partial fasting (n = 3), or (3) non-fasting (n = 2). We defined volumes of interest (VOIs), and generated organ time-activity curves (TACs). Organ radiation dosimetry was calculated using OLINDA/EXM v2.2 software.
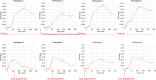
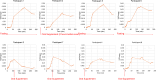
Results: There were no adverse events after 18F-VAT dosing. Radioactivity accumulated predominantly in the brain, hepatobiliary system, small intestine, bone, and urinary bladder. Across all fasting states, organ dosimetry revealed gallbladder as the critical organ (201.0 μSv/MBq) followed by liver (64.3 μSv/MBq), with a gender averaged effective dose of 17.5 ± 2.1 μSv/MBq (15.7 and 19.4 μSv/MBq for males and females, respectively.) Mean gallbladder time integrated activity significantly differed across non-fasting (36.6 MBq*h, 155.5 µSv/MBq), partial fasting (21.8 MBq*h, 107.6 µSv/MBq) and fasting PET acquisition (74.1 MBq*h, 270.5 µSv/MBq) (Kruskal-Wallis H 6.5, p = 0.04).
Conclusions: Human bio-distribution data showed high retention of 18F-VAT in the gallbladder and liver, where rat dosimetry studies do not accurately predict a safety profile given lack of gallbladder. Human dosimetry data appear different from fasting non-human primate data, indicating that up to 249 MBq (6.7 mCi) of 18F-VAT can be administered without exceeding a maximum dose to the gallbladder of 50 mSv (5 rem) without consideration of fasting state. Oral supplementation, administered just before and especially 90 min after 18F-VAT administration, accelerates gallbladder clearance. This reduces critical organ radiation exposure, allowing an administered dose of 18F-VAT to 465 MBq (12.6 mCi) in the optimal partial fasting state without exceeding a gallbladder dose of 50 mSv (5 rem).
Keywords: PET; Radiation dosimetry; VAChT; Vesicular acetylcholine transporter.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board and Human Research Protection Office of Washington University in St. Louis and in accordance with the Helsinki declaration and amendments. Signed informed consent was obtained for all human participants, approved by the Washington University Human Research Protection Office. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

References
Grants and funding
- R01 NS103988/NS/NINDS NIH HHS/United States
- NS075321/NH/NIH HHS/United States
- NS116025/NH/NIH HHS/United States
- NS103988/NH/NIH HHS/United States
- RF1 NS075321/NS/NINDS NIH HHS/United States
- R01 NS124789/NS/NINDS NIH HHS/United States
- U54 NS116025/NS/NINDS NIH HHS/United States
- NS075527/NH/NIH HHS/United States
- R01 NS075321/NS/NINDS NIH HHS/United States
- NS134586/NH/NIH HHS/United States
- R01 NS075527/NS/NINDS NIH HHS/United States
- R01 NS134586/NS/NINDS NIH HHS/United States
- NS124789/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources

