Therapeutic Burden as Predictor of Response to Baricitinib for Alopecia Areata in Real Life: Prospective Study
- PMID: 40622665
- PMCID: PMC12354933
- DOI: 10.1007/s13555-025-01468-1
Therapeutic Burden as Predictor of Response to Baricitinib for Alopecia Areata in Real Life: Prospective Study
Abstract
Introduction: Therapeutic burden (TB) has been proposed as a potential predictor of treatment outcomes in both dermatological and non-dermatological diseases. This study aims to introduce the concept in the context of alopecia areata (AA) and assess its potential value in supporting therapeutic decision-making in clinical practice.
Methods: A prospective cohort study was conducted including patients with AA who started treatment with baricitinib between January 2022 and January 2025 at a third-level hospital center. The main variable was TB, defined as the cumulative sum of previous systemic treatment cycles. An analysis was performed on whether socio-demographic or clinical factors were associated with TB.
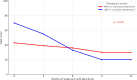
Results: Forty-four patients with AA treated with baricitinib were included. Most were women (65.90%) with a mean age of 37.70 (16.10) years. The predominant type of AA was multi-plaque (65.90%) and approximately one third (34.10%) had total/universal forms of the disease. Lower TB was statistically significantly associated with a greater reduction in Severity of Alopecia Tool (SALT) scores during the first 12 months of barictinib treatment compared with those patients with high TB (p < 0.05). This association was observed independently of all other known progression factors (duration of AA, baseline SALT, total/universal AA, female sex) (p < 0.05).
Conclusions: We present the concept of AA-adapted TB as a useful tool for categorizing patients with AA and contributing to therapeutic decision-making. Patients with AA with low TB showed a greater response to baricitinib treatment than patients who had received a greater number of systemic treatments previously.
Keywords: Alopecia Areata; Baricitinib; Janus kinase inhibitors; Therapeutic burden.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Daniel Muñoz-Barba, Alberto Soto-Moreno, Sofía Haselgruber-de Francisco, Manuel Sánchez-Díaz and Salvador Arias-Santiago have nothing to disclose. Ethical Approval: The current study was approved by the research ethics committee of Granada (code: SICEIA-2025-000370) and was performed in accordance with the principles of the Declaration of Helsinki. All patients gave informed consent before being included in the study.
Figures

References
-
- Konzett V, Smolen JS, Nash P, et al. Safety of Janus kinase inhibitors in immune-mediated inflammatory diseases-a systematic literature review informing the 2024 update of an international expert consensus statement. Ann Rheum Dis. 2025;84(5):697–715. - PubMed
LinkOut - more resources
Full Text Sources

