Multilevel Intervention and Human Papillomavirus Vaccination Disparities: A Secondary Analysis of a Cluster Randomized Trial
- PMID: 40622715
- PMCID: PMC12235494
- DOI: 10.1001/jamanetworkopen.2025.18895
Multilevel Intervention and Human Papillomavirus Vaccination Disparities: A Secondary Analysis of a Cluster Randomized Trial
Abstract
Importance: Uptake of human papillomavirus (HPV) vaccination varies by characteristics, exposing some children to higher HPV cancer risks than others.
Objective: To examine whether the effectiveness of a multilevel intervention on HPV vaccination differed by race and ethnicity, rurality, and Area Deprivation Index (ADI) in children ages 11 to 12 years.
Design, setting, and participants: A stepped-wedge cluster randomized trial was conducted from April 2018 to August 2022 among children at 6 Mayo Clinic primary care practices in Minnesota to improve HPV vaccination. This secondary analysis was performed from March to June 2024.
Intervention: A multilevel intervention that included parent reminder/recall letters, which alerted parents of children due or past due for vaccination, and health care professional audit/feedback reports, which alerted health care professionals of their own vaccination rates.
Main outcome and measure: Vaccine initiation (first dose of the 2-dose HPV vaccine) and vaccine completion (second dose) were the primary study outcomes. In this secondary analysis, the effect of the intervention on HPV vaccine initiation and completion by race and ethnicity, rurality, and ADI quartiles (Qs) was assessed.
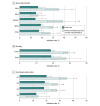
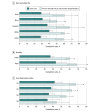
Results: A total of 6232 children aged 11 to 12 years (3285 [52.7%] male; 3481 [55.9%] aged 11 years and 2751 [44.1%] aged 12 years) were included in the analysis. Of the study participants, 304 (4.9%) were Asian, 561 (9.0%) Black, 146 (2.3%) Hispanic, 4501 (72.2%) White, and 720 (11.6%) other, including American Indian or Alaskan Native, Native Hawaiian or Pacific Islander, Other Pacific Islander, Samoan, unable to provide, unknown, chose not to disclose, or other unspecified. A total of 5434 participants (87.2%) were urban residents, and 2794 (44.8%) resided in ADI Q2 areas. With usual care, HPV vaccine initiation and completion rates were significantly lower with each increasing ADI quartile (initiation: Cochran-Armitage test for trend [SE], -0.02 [0.01]; P < .001; completion: Cochran-Armitage test for trend [SE], -0.05 [0.01]; P < .001) but did not differ by children's race and ethnicity or rurality. With the intervention, vaccine initiation increased significantly for most children (range of rates, 9.2% [95% CI, 5.2%-13.3%] to 24.0% [95% CI, 7.5%-40.6%]) except those with Black race, in rural settings, and in ADI Q4 (highest area deprivation); vaccine completion increased significantly for most children (range of rates, 19.4% [95% CI, 5.5%-33.3%] to 31.2% [95% CI, 12.1%-50.3%]) except for those in ADI Q4.
Conclusions and relevance: In this secondary analysis of a cluster randomized trial, a multilevel intervention was associated with increased HPV vaccination for most children but had limited effect for those residing in areas of highest deprivation. Future research should explore other intervention strategies that would effectively promote HPV vaccination among families in socioeconomically disadvantaged areas to reduce HPV vaccination disparities.
Trial registration: ClinicalTrials.gov Identifier: NCT03501992.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention . Cancers linked with HPV each year. Updated November 14, 2023. Accessed May 22, 2024. https://www.cdc.gov/cancer/hpv/cases.html?CDC_AAref_Val=https://www.cdc....
-
- Centers for Disease Control and Prevention. HPV vaccine safety and effectiveness. Updated November 16, 2021. Accessed May 29, 2025. https://www.cdc.gov/vaccines/vpd/hpv/hcp/safety-effectiveness.html
-
- Office of Disease Prevention and Health Promotion . Increase the proportion of adolescents who get recommended doses of the HPV vaccine — IID-08. Accessed March 11, 2024. https://odphp.health.gov/healthypeople/objectives-and-data/browse-object...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

