Passive immunotherapy for adults hospitalized with COVID-19: An individual participant data meta-analysis of six randomized controlled trials
- PMID: 40623115
- PMCID: PMC12282900
- DOI: 10.1371/journal.pmed.1004616
Passive immunotherapy for adults hospitalized with COVID-19: An individual participant data meta-analysis of six randomized controlled trials
Abstract
Background: Anti-SARS-CoV-2 monoclonal antibodies (mAb) reduce the risk of hospitalization in outpatients with mild-to-moderate COVID-19. However, the efficacy of treatment with mAbs and other passive immunotherapies in patients hospitalized with severe COVID-19 is not clear. The objective of this study was to assess the clinical effect of passive immunotherapy and its heterogeneity according to baseline endogenous neutralizing antibody status and SARS-CoV-2 antigen level, in adults hospitalized with SARS-CoV-2 infection and severe COVID-19.
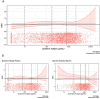
Methods and findings: We carried out a two-stage individual participant data meta-analysis of six double-blind, randomized, placebo-controlled trials conducted under the Therapeutics for Inpatients with COVID-19 (TICO) and the similarly designed Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) master protocols. Within each trial, three major outcomes (sustained recovery, mortality, and a composite safety outcome) were compared between treatment and placebo using Fine-Gray and Cox proportional hazards models. Trial-specific treatment differences for each of the three outcomes were pooled using a common effect meta-analysis. A total of 3,079 patients hospitalized for COVID-19 were enrolled in the six trials. Only 18% had received at least one dose of an anti-SARS-CoV-2 vaccine. Overall, the median plasma SARS-CoV-2 antigen level was 1,421 (IQR: 231-4,568) pg/mL, and 51% of patients were endogenous neutralizing antibody positive at study entry. The overall summary estimate of sustained recovery rate ratio (RRR) of the treatment versus placebo group was 1.06 (95% CI [0.99,1.14]), but this varied significantly by antibody serostatus. The RRR was 1.16 (95% CI [1.04,1.29]) among seronegative patients and 0.97 (95% CI [0.88,1.07]) in seropositive patients [p = 0.02 for interaction (the difference in RRR between seropositive and seronegative patients)]. The summary hazard ratio (HR) for mortality comparing treatment to placebo was 0.81 (95% CI [0.64,1.03]) overall, 0.69 (95% CI [0.50,0.95]) in seronegative patients, and 0.96 (95% CI [0.66,1.39]) in seropositive patients (interaction p = 0.18). There was no evidence that the treatment effect on any outcome differed according to antigen level, whether overall or within serostatus subgroups. In regards to the composite safety outcome, the overall summary HR comparing treatment group to placebo was 0.89 (95% CI [0.66,1.21]; Q = 3.47 [p = 0.63], I2 = 0.0%), and it was 0.83 (95% CI [0.70,0.99]) and 1.04 (95% CI [0.86,1.26]) in seronegative and seropositive patients, respectively. The main limitation of the methodology is that these results are limited to the analysis of the six trials in ACTIV-3/TICO and ITAC and are not intended to be a complete summary of all trials of passive immunotherapy.
Conclusions: Passive immunotherapy might be a useful treatment option for hospitalized patients with COVID-19 if administered before the appearance of endogenous antibodies. Development of similar passive immunotherapy could also be especially important during the early stages of a viral pandemic, or as novel viral variants emerge.
Copyright: © 2025 Knowlton et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: RLG served as a consultant for AbbVie, AstraZeneca, Eli Lilly, Gilead Sciences, Inc., GSK, Invivyd, Johnson & Johnson, Roche Pharmaceuticals, and Roivant Sciences; served as a national coordinating primary investigator for Johnson & Johnson; served on an academic steering committee for Roivant Sciences; received from Gilead Sciences, Inc., a gift in kind to Baylor Scott & White Research Institute to facilitate NCT03383419; owned de minimis stock in AbCellera Biologics; served as a speaker for Pfizer, outside the scope of COVID-19; and serves on the American Society of Transplant Surgeons Legislative & Regulatory Committee. GT has received grants unrelated to this study from Gilead Sciences Europe, UCL, EU, and National funds, all paid to her institution. Salary support for AB was also provided by the United Kingdom (Medical Research Council, grant MC_UU_00004/03 and MC_UU_00004/04).
Figures


References
-
- Dougan M, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, et al. A Randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. Clin Infect Dis. 2022;75(1):e440–9. doi: 10.1093/cid/ciab912 - DOI - PMC - PubMed
-
- ACTIV-3/TICO Bamlanivimab Study Group, Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, et al. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels : a randomized controlled trial. Ann Intern Med. 2022;175(2):234–43. doi: 10.7326/M21-3507 - DOI - PMC - PubMed
-
- ACTIV-3-Therapeutics for Inpatients with COVID-19 Study Group. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22(5):622–35. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

