Comorbidities and Their Influence on Outcomes and Infectious Complications in Autoimmune Encephalitis: A Multicenter Cohort Study
- PMID: 40623270
- PMCID: PMC12258818
- DOI: 10.1212/NXI.0000000000200434
Comorbidities and Their Influence on Outcomes and Infectious Complications in Autoimmune Encephalitis: A Multicenter Cohort Study
Abstract
Background and objectives: Comorbidities greatly influence the course of many diseases. However, systematic data on comorbidities in patients with autoimmune encephalitis (AE) are scarce. We aimed to characterize comorbidities in patients with common AE variants and assess their influence on outcome and occurrence of infectious complications.
Methods: This multicenter, retrospective cohort study analyzed adult patients with definite anti-N-methyl-d-aspartate receptor (NMDAR), anti-leucine-rich glioma-inactivated-1 (LGI1), anti-contactin-associated protein-like-2 (CASPR2), and anti-immunoglobulin-like cell adhesion molecule-5 (IgLON5) AE registered by the GErman NEtwork for REsearch on AuToimmune Encephalitis between June 2004 and July 2023. Preexisting conditions (PECs), secondary diagnoses, and infectious complications documented during hospitalization were analyzed. Outcome was evaluated using a modified Rankin Scale (mRS), with unfavorable outcome defined as mRS >2 after a minimum of 12 months of follow-up.
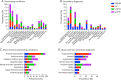
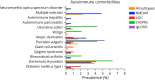
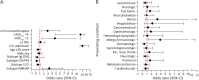
Results: Among 308 patients with AE (144 NMDAR-AE, 98 LGI1-AE, 47 CASPR2-AE, and 19 IgLON5-AE), nearly half had cardiovascular and metabolic/endocrine, one-third neurologic, and one-fifth psychiatric comorbidities. Accompanying autoimmunity was observed in 12.7%. Univariable analysis showed that the presence of ≥3 PECs (OR 2.80, 95% CI 1.57-4.92), especially cardiovascular (OR 1.93, 95% CI 1.09-3.30) and psychiatric PECs (OR 3.84, 95% CI 1.96-7.31), was associated with unfavorable outcome. Multivariable regression analysis confirmed psychiatric PECs as independent risk factors (OR 4.55, 95% CI 1.99-10.60). During hospitalization, 13.6% of patients developed severe infections, although these were not associated with unfavorable outcome (OR 1.94, 95% CI 0.97-3.89). AE disease severity (OR 5.41, 95% CI 1.38-27.67) and intensive care unit admission emerged as the only independent predictors of severe infections (OR 20.76, 95% CI 7.02-75.10).
Discussion: As premorbid psychiatric conditions are main factors associated with unfavorable outcomes, these patients would highly benefit from integrated interdisciplinary treatment centers, or at least heightened awareness of these factors. Concomitant autoimmunity affecting other organs is frequent and should be sought. The risk of severe infections during the acute phase of AE is moderate and, given their lack of effect on outcome, should not justify withholding appropriate immunotherapy, even in elderly patients with comorbidities. Future prognostic models should incorporate comorbidities, particularly psychiatric ones, to enhance risk assessment and guide personalized care strategies.
Conflict of interest statement
A. Bohn received travel and speaker honoraria from Merck and Roche; K. Angstwurm received travel support (till 2014) from Alexion, Bayer, BiogenIdec, MerckSerono, Novartis, and Teva and honoria from Biogen and TEVA, he supported neuroimmunologic studies for Alexion, Bayer, BiogenIdec, MerckSerono, Roche, and Novartis; C.G. Bien, K. Doppler, L. Ehmke, J. Havla, F. Hoffmann, D. Hudasch, and J. Klausewitz report no disclosures relevant to the manuscript; F.F. Konen reports honoraria and travel grants from Argenx, Alexion, Merck, and Novartis and is a DFG-funded (Deutsche Forschungsgesellschaft) and MHH-funded (Hannover Medical School) fellow of the Clinician Scientist Program PRCATIS at MHH; M. Korporal-Kuhnke and A. Kraft report no disclosures relevant to the manuscript; T. Kümpfel has received speaker honoraria and/or personal fees for advisory boards from Novartis Pharma, Roche Pharma, Alexion/Astra Zeneca, Horizon Therapeutics/Amgen, Merck, Chugai Pharma, and Biogen, the institution she works for has received compensation for serving as a member of a steering committee from Roche, she is a site principal investigator in several randomized clinical trials (Novartis Pharma, Roche Pharma, BMS, and Sanofi Genzyme) and in a randomized clinical trials supported by the BMBf (funding code: 01 GM1908E), and her institution has received compensation for clinical trials all outside the present work; F. Leypoldt was also supported by E-Rare Joint Transnational research support (ERA-Net, LE3064/2-1), Stiftung Pathobiochemie of the German Society for Laboratory Medicine, and HORIZON MSCA 2022 Doctoral Network 101119457—IgG4-TREAT and discloses speaker honoraria from Grifols, Teva, Biogen, Bayer, Roche, Novartis, and Fresenius, travel funding from Merck, Grifols, and Bayer, and serving on advisory boards for Roche, Biogen, and Alexion; M. Madlener, L.K. Pfeffer, S. Pfeuffer, D. Pul, A. Rada, S. Rauer, C. Sänger, T. Seifert-Held, K.-W. Sühs, F.S. Thaler, T. Tsaktanis, B. Vlad, and K.P. Wandinger report no disclosures relevant to the manuscript; J. Wickel reports honoraria from Argenx and research support from Ionis Pharmaceuticals; S.C. Tauber served on the scientific advisory board for Roche and Merck and received travel and speaker honoraria from Novartis, Teva, Merck, Roche, and Biogen. Full disclosure form information provided by the authors is available with the full text of this article at
Figures


References
-
- Schwarz L, Akbari N, Prüss H, Meisel A, Scheibe F. Clinical characteristics, treatments, outcome, and prognostic factors of severe autoimmune encephalitis in the intensive care unit: standard treatment and the value of additional plasma cell-depleting escalation therapies for treatment-refractory patients. Eur J Neurol. 2023;30(2):474-489. doi: 10.1111/ene.15585 - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
