Precision targeting of rhabdomyosarcoma by combining primary CAR NK cells and radiotherapy
- PMID: 40623716
- PMCID: PMC12232460
- DOI: 10.1136/jitc-2024-011330
Precision targeting of rhabdomyosarcoma by combining primary CAR NK cells and radiotherapy
Abstract
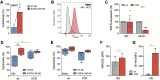
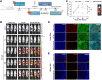
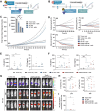
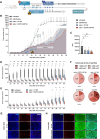
Background: Rhabdomyosarcoma (RMS) is the most common type of soft-tissue sarcoma in children, and it remains a challenging cancer with poor outcomes in high-risk and metastatic patients. This study reports the use of epidermal growth factor receptor (EGFR)-targeted chimeric antigen receptor (CAR) natural killer (NK) cells in combination with radiotherapy as a novel immunotherapeutic approach for RMS treatment.Methods: Primary human NK cells from healthy donors were engineered using lentiviral transduction to express a cetuximab-based EGFR-specific CAR. The ability of the engineered NK cells to lyse RMS cells was then assessed in vitro in RMS monolayers and spheroids, as well as against chemotherapy-resistant and primary patient-derived RMS cells. Migratory properties of NK cells were observed in a subcutaneous RMS xenograft model using in vivo imaging, and the efficacy of EGFR-CAR NK cells in combination with localized fractionated radiotherapy was analyzed.Results: Primary human EGFR-CAR NK cells demonstrated enhanced cytotoxicity against multiple RMS cell lines in both two-dimensional culture and three-dimensional spheroid models. Furthermore, EGFR-CAR NK cells were highly efficient against chemotherapy-resistant RMS cells and patient-derived samples. Importantly, EGFR-CAR NK cells also exhibited improved tumor homing compared with non-transduced NK cells in an in vivo RMS xenograft model. Notably, the combination of EGFR-CAR NK cell therapy with fractionated radiotherapy further enhanced NK cell infiltration into the tumor and reduced tumor growth.Conclusion: This study provides a proof-of-concept for EGFR-CAR NK cells as a promising immunotherapy for RMS, particularly when combined with radiotherapy to overcome barriers of solid tumors. This combinatorial approach may hold potential to improve outcomes for patients with RMS and other EGFR-expressing malignancies.
Keywords: Chimeric antigen receptor - CAR; Immunotherapy; Natural killer - NK; Radiotherapy/radioimmunotherapy; Solid tumor.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: EU has a sponsored research project with Gilead and BMS and acts as a medical advisor for Phialogics and CRIION. HB has obtained research support from Bayer, Chugai, Erydel, Fresenius, Miltenyi, Sandoz-Hexal (a Novartis Company), Terumo BCT, honoraria or speakers‘ fees from BMS/Celgene, Fresenius, Kiadis, Miltenyi, Novartis, has served as consultant or on advisory boards of Apriligen, Arensia, Boehringer Ingelheim Vetmed, BMS/Celgene, Editas, medac, NMDP, Novartis, Sandoz-Hexal, receives royalties from medac and owns stock from Healthineers. The other authors have no competing interests to declare.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
