GRACE: protocol for a UK, secondary care, multicentre, assessor-blinded randomised controlled trial with a non-inferiority comparison to evaluate graduated compression stockings as an adjunct to extended duration pharmacological thromboprophylaxis for venous thromboembolism prevention
- PMID: 40623749
- PMCID: PMC12230952
- DOI: 10.1136/bmjopen-2024-095482
GRACE: protocol for a UK, secondary care, multicentre, assessor-blinded randomised controlled trial with a non-inferiority comparison to evaluate graduated compression stockings as an adjunct to extended duration pharmacological thromboprophylaxis for venous thromboembolism prevention
Abstract
Introduction: Venous thromboembolism (VTE) occurs when a blood clot forms in a vein. It is comprised of deep vein thrombosis (DVT) and pulmonary embolism and can be potentially life-threatening. Patients undergoing surgery are at increased risk of developing VTE within hospital admission and 90 days after hospital discharge are collectively known as hospital-acquired thrombosis (HAT). Without the use of thromboprophylaxis, the untreated risk of VTE is reported to be as high as 40-60% in those undergoing major orthopaedic procedures and around 15-40% in the general surgical population.HAT accounts for around 12 000 deaths per year in the UK. For patients undergoing surgery, there is good evidence for the use of thromboprophylaxis to prevent VTE.Thromboprophylaxis is available in both pharmacological and mechanical forms. While there is a huge body of evidence demonstrating that pharmacological thromboprophylaxis significantly reduces VTE by 30-65%, the benefit of graduated compression stockings (GCS) has been called into question. The GRACE study (Graduated Compression stocking as an adjunct to Extended duration pharmacological thromboprophylaxis for venous thromboembolism prevention) aims to evaluate the adjuvant benefit of GCS in addition to extended duration pharmacological thromboprophylaxis (EDPTP) for elective surgical patients at highest risk of VTE.
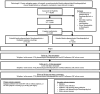
Methods and analysis: GRACE is a pragmatic, multicentre randomised trial of adults undergoing surgery who are at high risk of VTE. Participants are randomised into a 1:1 ratio to either EDPTP and compression stockings (control arm) or EDPTP (intervention arm). Following randomisation, participants will undergo surgery and be followed up centrally at 7, 21-35 and 90 days after their procedure. All participants will be offered a bilateral full lower limb duplex scan at 21-35 days post procedure to capture any asymptomatic DVT.The trial aims to randomise 8608 participants from around 50 National Health Service (NHS) and non-NHS sites in the UK over a 24-month period. The primary endpoint is any imaging-confirmed incidence of VTE within 90 days of surgery.
Ethics and dissemination: On 20 December 2023, GRACE received favourable ethical approval from the Wales Research Ethics Committee 3 Cardiff (23/WA/0350) and the Health Research Authority (IRAS 333539). The results of the study will be disseminated via peer-reviewed publications, presentation at national and international conferences and to study participants via electronic newsletter and social media channels.
Trial registration number: ISRCTN11667770.
Keywords: HAEMATOLOGY; SURGERY; Thromboembolism.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: BJH is cochair of the trustee board for Thrombosis UK and does not receive any monies for this role. SS received Research grant and PhD tuition fees made to Imperial College London from Actegy and has received support for attending meetings and/or travel from Imperial College London. AD and JS received National Institute for Health Research grant funding to Imperial College London for the GRACE trial. JS is in receipt of British Heart Foundation funding for other clinical studies. JS has unpaid roles at Circulation Foundation (charity), Vascular Society Research Special Interest Groups and Research Committee, Council Member, Surgical Research Society & Vascular and Endovascular Research Network. JS is an employee at Cleveland Clinic London. All other authors have no competing interest to declare.
Figures
Similar articles
-
Pentasaccharides for the prevention of venous thromboembolism.Cochrane Database Syst Rev. 2016 Oct 31;10(10):CD005134. doi: 10.1002/14651858.CD005134.pub3. Cochrane Database Syst Rev. 2016. PMID: 27797404 Free PMC article.
-
Neuromuscular electrical stimulation for the prevention of venous thromboembolism.Cochrane Database Syst Rev. 2017 Nov 21;11(11):CD011764. doi: 10.1002/14651858.CD011764.pub2. Cochrane Database Syst Rev. 2017. PMID: 29161465 Free PMC article.
-
Interventions for implementation of thromboprophylaxis in hospitalized patients at risk for venous thromboembolism.Cochrane Database Syst Rev. 2018 Apr 24;4(4):CD008201. doi: 10.1002/14651858.CD008201.pub3. Cochrane Database Syst Rev. 2018. PMID: 29687454 Free PMC article.
-
Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism.Cochrane Database Syst Rev. 2013 Jul 16;(7):CD008201. doi: 10.1002/14651858.CD008201.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Apr 24;4:CD008201. doi: 10.1002/14651858.CD008201.pub3. PMID: 23861035 Updated.
-
Primary prophylaxis for venous thromboembolism in people undergoing major amputation of the lower extremity.Cochrane Database Syst Rev. 2013 Dec 16;(12):CD010525. doi: 10.1002/14651858.CD010525.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jul 21;7:CD010525. doi: 10.1002/14651858.CD010525.pub3. PMID: 24343728 Updated.
References
-
- NHS digital 5.1 deaths from venous thromboembolism (VTE) related events within 90 days post discharge from hospital (2007-2019) 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
