Development and retrospective validation of an artificial intelligence system for diagnostic assessment of prostate biopsies: study protocol
- PMID: 40623883
- PMCID: PMC12258300
- DOI: 10.1136/bmjopen-2024-097591
Development and retrospective validation of an artificial intelligence system for diagnostic assessment of prostate biopsies: study protocol
Abstract
Introduction: Histopathological evaluation of prostate biopsies using the Gleason scoring system is critical for prostate cancer diagnosis and treatment selection. However, grading variability among pathologists can lead to inconsistent assessments, risking inappropriate treatment. Similar challenges complicate the assessment of other prognostic features like cribriform cancer morphology and perineural invasion. Many pathology departments are also facing an increasingly unsustainable workload due to rising prostate cancer incidence and a decreasing pathologist workforce coinciding with increasing requirements for more complex assessments and reporting. Digital pathology and artificial intelligence (AI) algorithms for analysing whole slide images show promise in improving the accuracy and efficiency of histopathological assessments. Studies have demonstrated AI's capability to diagnose and grade prostate cancer comparably to expert pathologists. However, external validations on diverse data sets have been limited and often show reduced performance. Historically, there have been no well-established guidelines for AI study designs and validation methods. Diagnostic assessments of AI systems often lack preregistered protocols and rigorous external cohort sampling, essential for reliable evidence of their safety and accuracy.
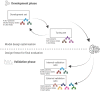
Methods and analysis: This study protocol covers the retrospective validation of an AI system for prostate biopsy assessment. The primary objective of the study is to develop a high-performing and robust AI model for diagnosis and Gleason scoring of prostate cancer in core needle biopsies, and at scale evaluate whether it can generalise to fully external data from independent patients, pathology laboratories and digitalisation platforms. The secondary objectives cover AI performance in estimating cancer extent and detecting cribriform prostate cancer and perineural invasion. This protocol outlines the steps for data collection, predefined partitioning of data cohorts for AI model training and validation, model development and predetermined statistical analyses, ensuring systematic development and comprehensive validation of the system. The protocol adheres to Transparent Reporting of a multivariable prediction model of Individual Prognosis Or Diagnosis+AI (TRIPOD+AI), Protocol Items for External Cohort Evaluation of a Deep Learning System in Cancer Diagnostics (PIECES), Checklist for AI in Medical Imaging (CLAIM) and other relevant best practices.
Ethics and dissemination: Data collection and usage were approved by the respective ethical review boards of each participating clinical laboratory, and centralised anonymised data handling was approved by the Swedish Ethical Review Authority. The study will be conducted in agreement with the Helsinki Declaration. The findings will be disseminated in peer-reviewed publications (open access).
Keywords: Artificial Intelligence; HISTOPATHOLOGY; Prostate; Retrospective Studies.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: NM, LE, KK and ME are co-founders and shareholders of Clinsight AB, and MR is a co-founder and shareholder of Stratipath AB. All other authors have no competing interests to declare.
Figures
Similar articles
-
MRI software and cognitive fusion biopsies in people with suspected prostate cancer: a systematic review, network meta-analysis and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(61):1-310. doi: 10.3310/PLFG4210. Health Technol Assess. 2024. PMID: 39367754 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Artificial intelligence for detecting keratoconus.Cochrane Database Syst Rev. 2023 Nov 15;11(11):CD014911. doi: 10.1002/14651858.CD014911.pub2. Cochrane Database Syst Rev. 2023. PMID: 37965960 Free PMC article.
-
The measurement and monitoring of surgical adverse events.Health Technol Assess. 2001;5(22):1-194. doi: 10.3310/hta5220. Health Technol Assess. 2001. PMID: 11532239
-
A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases.Br J Dermatol. 2024 Jul 16;191(2):261-266. doi: 10.1093/bjd/ljae142. Br J Dermatol. 2024. PMID: 38581445
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
