[Analysis of the association between pre- and post-treatment genetic mutation status and treatment efficacy and survival in patients with newly diagnosed myelodysplastic syndromes with excess blasts receiving hypomethylating agent therapy]
- PMID: 40623900
- PMCID: PMC12268287
- DOI: 10.3760/cma.j.cn121090-20241210-00553
[Analysis of the association between pre- and post-treatment genetic mutation status and treatment efficacy and survival in patients with newly diagnosed myelodysplastic syndromes with excess blasts receiving hypomethylating agent therapy]
Abstract
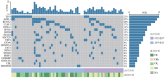
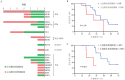
Objective: To investigate the association between pre- and post-treatment gene mutation profiles and clinical outcomes (treatment response and prognosis) in patients with myelodysplastic syndromes with excess blasts (MDS-EB) receiving hypomethylating agent (HMA) monotherapy. Methods: The clinical characteristics, treatment efficacy, and survival outcomes of 69 treatment-naive patients with MDS-EB who underwent next-generation sequencing (NGS) before treatment and completed at least 4 cycles of HMA monotherapy at the Institute of Hematology & Blood Diseases Hospital, CAMS & PUMC, between June 2016 and September 2023, were retrospectively analyzed. Results: ① The cohort comprised 47 males and 22 females with a median age of 62 years (range: 41-80). Thirty-nine patients were classified as MDS-EB1 and 30 as MDS-EB2. The median number of treatment cycles was 6 (range: 4-35). The median follow-up duration was 22 months (range: 5-72), and the median overall survival (OS) was 32 months (95% CI: 27-43). ② The presence of DTA (DNMT3A, TET2, or ASXL1) mutations, signaling pathway mutations, transcription factor mutations, or splicing factor mutations before HMA treatment showed no significant association with the best response within 4 treatment cycles, duration of response (DOR), or OS. TP53 mutation status was significantly associated with DOR and shorter OS. The median DOR was 3 months (95% CI: 1-10) for patients with biallelic TP53 mutations, 10 months (95% CI: 3-34) for those with monoallelic TP53 mutations, and 16 months (95% CI: 8-27) in patients without TP53 mutations (P=0.032). The median OS was 16 months (95% CI: 7-38), 15 months (95% CI: 6-40), and 35 months (95% CI: 14-91), respectively (P<0.001). ③ Neither the Revised International Prognostic Scoring System (IPSS-R) nor the Molecular International Prognostic Scoring System (IPSS-M) could predict the best response within 4 treatment cycles or DOR in patients receiving HMA therapy. ④ Among patients without TP53 mutations, the median OS was 55 months (95% CI: 9-106) for the major clone significant clearance group (n=14) and 31 months (95% CI: 16-184) for the major clone non-significant clearance group (n=10) (P=0.013). For patients who responded to HMA treatment and had significant major clone clearance, the 3-year OS rate reached (77.8±13.9) %. Conclusion: For MDS-EB patients receiving HMA monotherapy, single gene mutations, IPSS-R, and IPSS-M could not effectively predict treatment outcomes before therapy. However, for patients without TP53 mutations, monitoring the degree of major clone clearance by NGS during treatment may predict the long-term efficacy in MDS patients receiving HMA therapy.
目的: 探索接受去甲基化药物(HMA)单药治疗的骨髓增生异常综合征伴原始细胞增多(MDS-EB)患者治疗前后基因突变状态与疗效及预后的关系。 方法: 2016年6月至2023年9月于中国医学科学院血液病医院完成了至少4个疗程HMA单药治疗且治疗前进行了二代测序(NGS)的69例初治MDS-EB患者,回顾性分析其临床特征、疗效、生存时间以及生存情况。比较HMA治疗前后基因突变症状对疗效与预后的影响。 结果: ①69例患者中,男47例,女22例,中位年龄62(41~80)岁。MDS-EB1 39例,MDS-EB2 30例,中位治疗6(4~35)个疗程。中位随访22(5~72)个月,中位生存期32(95%CI:27~43)个月。②HMA治疗前是否伴有DTA(DNMT3A、TET2和ASXL1)突变、信号转导基因突变、转录因子突变或剪接因子突变与4个疗程内最佳疗效、疗效持续时间(DOR)和总生存(OS)均无显著相关性。TP53突变状态与DOR相关且OS时间显著缩短,伴有双打击TP53突变者、单打击TP53突变者和非TP53突变者的中位DOR分别为3(95%CI:1~10)、10(95%CI:3~34)和16(95%CI:8~27)个月(P=0.032),中位OS时间分别为16(95%CI:7~38)、15(95%CI:6~40)和35(95%CI:14~91)个月(P<0.001)。③修订的国际预后积分系统(IPSS-R)和含分子遗传学指标的国际预后积分系统(IPSS-M)均不能预测接受HMA治疗4个疗程内最佳疗效和DOR。④非TP53突变患者,主克隆显著清除组(14例)和主克隆非显著清除组(10例)中位OS时间分别为55(95%CI:9~106)个月和31(95%CI:16~184)个月(P=0.013)。HMA治疗有效且主克隆显著清除者的3年OS率达(77.8±13.9)%。 结论: MDS-EB患者接受HMA单药治疗前,单一基因突变、IPSS-R和IPSS-M预后积分系统均无法有效预测治疗效果。非TP53突变患者,通过NGS监测治疗过程中主克隆清除程度可预测接受HMA治疗的MDS患者的长期疗效。.
Keywords: Efficacy; Hypomethylating agents; Major clones; Myelodysplastic syndromes; Overall survival.
Conflict of interest statement
Figures
Similar articles
-
Allogeneic Hematopoietic Cell Transplantation Improves Outcome in Myelodysplastic Syndrome Across High-Risk Genetic Subgroups: Genetic Analysis of the Blood and Marrow Transplant Clinical Trials Network 1102 Study.J Clin Oncol. 2023 Oct 1;41(28):4497-4510. doi: 10.1200/JCO.23.00866. Epub 2023 Aug 22. J Clin Oncol. 2023. PMID: 37607457 Free PMC article.
-
Evidence-based risk stratification of myeloid neoplasms harboring TP53 mutations.Blood Adv. 2025 Jul 8;9(13):3370-3380. doi: 10.1182/bloodadvances.2024015238. Blood Adv. 2025. PMID: 40085954 Free PMC article.
-
Early identification of TP53 mutations and TP53 allelic state in myelodysplastic neoplasms and acute myeloid leukemia via point-of-care p53 immunohistochemistry.Cancer. 2025 Jul 1;131(13):e35950. doi: 10.1002/cncr.35950. Cancer. 2025. PMID: 40542737
-
Thrombopoietin mimetics for patients with myelodysplastic syndromes.Cochrane Database Syst Rev. 2017 Sep 30;9(9):CD009883. doi: 10.1002/14651858.CD009883.pub2. Cochrane Database Syst Rev. 2017. PMID: 28962071 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous