[Establishment of a chronic lymphocytic leukemia mouse model via adoptive transfer of Eμ-TCL1 transgenic splenocytes]
- PMID: 40623904
- PMCID: PMC12268288
- DOI: 10.3760/cma.j.cn121090-20241120-00461
[Establishment of a chronic lymphocytic leukemia mouse model via adoptive transfer of Eμ-TCL1 transgenic splenocytes]
Abstract
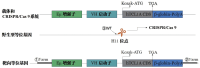
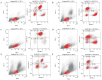
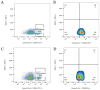
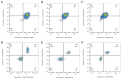
Objective: To generate a chronic lymphocytic leukemia (CLL) mouse model with intact immune competence and short latency by adoptively transferring (AT) splenocytes from immunoglobulin heavy-chain enhancer-driven T-cell leukemia/lymphoma 1 (Eμ-TCL1) transgenic donors into wild-type (WT) recipients. Methods: Specific pathogen-free C57BL/6J WT mice and H11-Eμ-VH-TCL1-β-globin-PolyA knock-in mice were utilized. The H11-Eμ-VH-TCL1-β-globin-PolyA knock-in mice were generated using CRISPR/Cas9 technology, and their genotypes were confirmed by PCR. Experimental animals were randomly divided into an adoptive transfer (AT) group and a WT control group (n=10 per group). Mice in the AT group received an intraperitoneal injection of splenocytes from H11-Eμ-VH-TCL1-β-globin-PolyA knock-in mice. The weight and general condition of the mice were monitored. Mice were euthanized by cervical dislocation at 9 weeks post-transplantation. The CLL model was validated using key indicators, including pathological manifestations, changes in peripheral blood leukocyte counts, and immunophenotype. Results: AT group mice exhibited significantly increased spleen weight [ (0.92±0.16) g vs (0.06±0.01) g in WT group, P<0.05] and liver weight [ (2.11±0.56) g vs (1.42±0.13) g in WT group, P=0.006], indicative of marked splenomegaly and hepatomegaly. The peripheral blood leukocyte count was significantly higher in the AT group [ (124.33±8.74) ×10(9)/L] compared to the WT group [ (5.55±1.67) ×10(9)/L] (P=0.002). Similarly, the percentage of peripheral blood B lymphocytes was markedly increased in the AT group versus the WT group [ (69.13±6.88) % vs (39.78±5.94) %, P<0.05]. Histopathological examination revealed CLL manifestations in the spleen, lymph nodes, and bone marrow of AT group mice, with significant lymphocytic infiltration observed in the liver, lung, and kidney tissues. Flow cytometry analysis showed that the percentages of CD19(+)CD5(+) B lymphocytes among total lymphocytes in peripheral blood, bone marrow, and spleen of the AT group were (61.37±9.92) %, (28.61± 7.08) %, and (86.03±5.78) %, respectively. These were significantly higher (all P<0.05) than in the WT group [ (4.51±1.32) %, (5.58±1.46) %, and (14.33±3.20) %]. Furthermore, these CLL-like cells in the AT group were positive for CD43 and CD200, but showed lower expression of CD20, CD22, and CD79b compared to WT B cells. Conclusion: Adoptive transfer of splenocytes from Eμ-TCL1 transgenic mice successfully established a CLL mouse model with a relatively short latency period. This model represents a valuable preclinical tool for investigating CLL and related pathologies.
目的: 利用免疫球蛋白重链增强子调控的T细胞白血病/淋巴瘤1(Eμ-TCL1)转基因小鼠脾细胞过继性转移(AT)至野生型(WT)小鼠体内,建立一种具有自身免疫功能、成模周期较短的慢性淋巴细胞白血病(CLL)小鼠模型。 方法: 实验使用了无特定病原体(SPF)级健康的C57BL/6J WT小鼠和H11-Eμ-VH-TCL1-β-globin-PolyA基因敲入小鼠。通过CRISPR/Cas9技术构建了H11-Eμ-VH-TCL1-β-globin-PolyA基因敲入小鼠,并采用PCR方法鉴定小鼠的基因型。实验动物被随机分为AT组和WT组,每组10只,AT组为H11-Eμ-VH-TCL1-β-globin-PolyA基因敲入小鼠脾细胞腹腔注射至体内的WT小鼠。监测小鼠的体重和一般情况,并在移植后第9周进行颈椎脱臼处死。通过病理学表现、外周血白细胞变化和免疫表型等关键指标对CLL小鼠模型进行疾病验证。 结果: AT组脾重量为(0.92±0.16)g、肝重量为(2.11±0.56)g;WT组脾重量为(0.06±0.01)g、肝重量为(1.42±0.13)g,AT组出现明显的肝(P=0.006)、脾(P<0.05)肿大。AT组外周血白细胞数量较WT组明显增多[(124.33±8.74)×10(9)/L对(5.55±1.67)×10(9)/L,P=0.002];AT组外周血B淋巴细胞百分比较WT组增多[(69.13±6.88)%对(39.78±5.94)%,P<0.05]。病理组织学检查发现AT组小鼠的脾、淋巴结、骨髓出现CLL病理表现,肝、肺、肾组织均有明显淋巴细胞浸润。流式细胞术检测结果示AT组CD19(+)CD5(+) B淋巴细胞占淋巴细胞百分比在外周血、骨髓和脾中分别为(61.37±9.92)%、(28.61±7.08)%、(86.03±5.78)%;WT组CD19(+)CD5(+) B淋巴细胞占淋巴细胞百分比在外周血、骨髓和脾中分别为(4.51±1.32)%、(5.58±1.46)%、(14.33±3.2)%;AT组外周血、骨髓、脾中CD19(+)CD5(+) B淋巴细胞占比较WT组增多,差异均具有统计学意义(均P<0.05),且CD43、CD200表达阳性,但CD20、CD22、CD79b的表达低于WT组。 结论: 通过Eμ-TCL1转基因小鼠脾细胞AT,成功建立了成型周期相对较短的CLL小鼠模型,其可作为一种理想的临床前模型用于CLL相关疾病研究。.
Keywords: CD19; CD5; Leukemia, lymphocytic, chronic, B-cell; Models, animal.
Conflict of interest statement
Figures




Similar articles
-
BET Protein Inhibition Relieves MDSC-Mediated Immune Suppression in Chronic Lymphocytic Leukemia.Hemato. 2025 Jun;6(2):14. doi: 10.3390/hemato6020014. Epub 2025 May 24. Hemato. 2025. PMID: 40708600 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Mixed-methods research to support the use of new lymphoma-specific patient-reported symptom measures derived from the EORTC item library.J Patient Rep Outcomes. 2024 Jan 22;8(1):8. doi: 10.1186/s41687-024-00683-2. J Patient Rep Outcomes. 2024. PMID: 38252198 Free PMC article. Review.
-
Trichinella spiralis adult excretory-secretory antigen promotes peripheral regulatory T cell differentiation and attenuates experimental colitis via TGF-β-like mechanisms.Parasit Vectors. 2025 Jul 1;18(1):240. doi: 10.1186/s13071-025-06877-x. Parasit Vectors. 2025. PMID: 40597393 Free PMC article.
-
Eμ-TCL1 adoptive transfer mouse model of chronic lymphocytic leukemia.Methods Cell Biol. 2024;188:109-129. doi: 10.1016/bs.mcb.2024.03.012. Epub 2024 Apr 18. Methods Cell Biol. 2024. PMID: 38880520
References
-
- 付 春玲, 龚 艳青, 万 艳, et al. 应用MEC-1与HG3细胞株建立慢性淋巴细胞白血病BALB/c裸鼠皮下移植瘤的模型[J] 中国实验血液学杂志. 2016;24(6):1644–1648. doi: 10.7534/j.issn.1009-2137.2016.06.006. - DOI - PubMed
- Fu CL, Gong YQ, Wan Y, et al. Establishment of human chronic lymphocytic leukemia model in the BALB/c nude mice by using MEC-1 and HG3 cell lines[J] J Experi Hematol. 2016;24(6):1644–1648. doi: 10.7534/j.issn.1009-2137.2016.06.006. - DOI - PubMed
-
- 赵 子璇, 南 苗苗, 贺喜 白乙, et al. MEC-1移植法构建慢性淋巴细胞白血病小鼠模型[J] 中国实验动物学报. 2023;31(1):75–81. doi: 10.3969/j.issn.1005-4847.2023.01.009. - DOI
- Zhao ZX, Nan MM, Hexi Bayar, et al. Preparation of mouse models of chronic lymphocytic leukemia by MEC-1 cells transplantation[J] Acta Laboratorium Animalis Scientia Sinica. 2023;31(1):75–81. doi: 10.3969/j.issn.1005-4847.2023.01.009. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 81860033, 8216010085/National Natural Science Foundation of China
- TSYC202301A006/Xinjiang Uygur Autonomous Region Tianshan Yingcai Medical and Health High-level Talent Training Project
- 2022D14008/Xinjiang Uygur Autonomous Region "Tianshan Innovation Team Program" Project
- The Second Batch of the Tianchi Talent Introduction Program in the Autonomous Region
LinkOut - more resources
Full Text Sources

