eEF2K is a poor prognostic factor and novel molecular target in pancreatic cancer: regulating tumor growth and progression via the tumor microenvironment
- PMID: 40624025
- PMCID: PMC12234705
- DOI: 10.1038/s41419-025-07803-w
eEF2K is a poor prognostic factor and novel molecular target in pancreatic cancer: regulating tumor growth and progression via the tumor microenvironment
Abstract
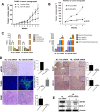
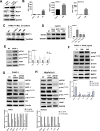
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers, with an average survival time of only six months following diagnosis, even with currently available therapies. Thus, PDAC represents a significant therapeutic challenge, necessitating a deeper understanding of its biology and tumor microenvironment (TME) to develop more effective treatments and improve patient outcomes. Here, we report that the expression of Eukaryotic Elongation Factor-2 Kinase (eEF2K) is associated with shorter patient survival and demonstrate that eEF2K signaling is critical for the PDAC tumor growth and regulated by the TME. Furthermore, in vivo targeted genetic inhibition of eEF2K suppressed tumor growth in two different PDAC mouse models, reduced tumor-associated macrophages (TAMs), and induced marked apoptosis in tumor tissues without any signs of toxicity. Our data suggest that eEF2K knockdown diminishes the activity of the AXL receptor tyrosine kinase and reduces the expression of macrophage-derived factors, such as Monocyte Chemoattractant Protein-1 (MCP1), along with the Gas6/AXL signaling pathway in PDAC cells. Additionally, analysis of the NCI-TCGA PDAC patient database further showed that eEF2K expression, in the presence of TAM markers, correlates with even shorter patient survival. TAM-released factors, such as MCP1, Gas6, and exosomes, induce eEF2K expression in PDAC cells, as well as the activity of AXL, SRC, VEGF, Snail, and MMP2, contributing to epithelial-to-mesenchymal transition (EMT), invasion, metastasis, and angiogenesis. In conclusion, our findings reveal for the first time that eEF2K is a critical oncogenic driver of PDAC tumor growth and thus targeting eEF2K represents a promising and novel therapeutic strategy for PDAC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Animal experiments were performed in accordance with institutional guidelines and regulations. The animal study protocol was approved by the MD Anderson Cancer Center Animal Research Ethics Committee. Written informed consent was obtained from participants for the publication of identifiable images included in the manuscript.
Figures




Similar articles
-
β-Catenin mediated TAM phenotype promotes pancreatic cancer metastasis via the OSM/STAT3/LOXL2 axis.Neoplasia. 2025 Feb;60:101096. doi: 10.1016/j.neo.2024.101096. Epub 2024 Dec 30. Neoplasia. 2025. PMID: 39740539 Free PMC article.
-
PLAGL2 as a prognostic biomarker and an EMT-promoting factor in PDAC.Sci Rep. 2025 Jul 14;15(1):25425. doi: 10.1038/s41598-025-09591-x. Sci Rep. 2025. PMID: 40659704 Free PMC article.
-
TYROBP overexpression alters macrophage phenotype and enhances pancreatic cancer stemness through STAT3 and PKM2 signaling.Cell Signal. 2025 Oct;134:111949. doi: 10.1016/j.cellsig.2025.111949. Epub 2025 Jun 18. Cell Signal. 2025. PMID: 40553966
-
The type I collagen paradox in PDAC progression: microenvironmental protector turned tumor accomplice.J Transl Med. 2025 Jul 4;23(1):744. doi: 10.1186/s12967-025-06778-8. J Transl Med. 2025. PMID: 40616159 Free PMC article. Review.
-
Roles of lncRNAs in pancreatic ductal adenocarcinoma: Diagnosis, treatment, and the development of drug resistance.Hepatobiliary Pancreat Dis Int. 2023 Apr;22(2):128-139. doi: 10.1016/j.hbpd.2022.12.002. Epub 2022 Dec 14. Hepatobiliary Pancreat Dis Int. 2023. PMID: 36543619
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Nagendram S, Bhattacharya S. Pancreatic cancer: a glimmer of hope. Trends Urol Men’s Health. 2023;14:5–10.
-
- Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

