Targeting dysregulated molecular pathways in cancer cell lines using small molecule inhibitors as a promising therapeutic strategy
- PMID: 40624082
- PMCID: PMC12234987
- DOI: 10.1038/s41598-025-06892-z
Targeting dysregulated molecular pathways in cancer cell lines using small molecule inhibitors as a promising therapeutic strategy
Abstract
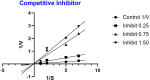
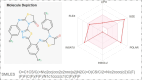
Biologically active heterocycles hold considerable potential in modulating cellular pathways associated with cancer progression. The present study focuses on the design and synthesis of novel thiadiazole-thiazolidinone hybrid scaffolds aimed at inhibiting the proliferation of cancer cells. The synthesized compounds were evaluated for their cytotoxic efficacy against human cancer cell lines, including HepG2 (hepatocellular carcinoma), MCF-7 (breast adenocarcinoma), HCT-116 (colorectal carcinoma), and W138 (lung fibroblast-derived carcinoma). Doxorubicin was used as a reference standard. Among the synthesized library, compound 7 demonstrated the most potent antiproliferative activity across all tested cell lines, indicating its potential role in targeting molecular pathways involved in tumor growth and survival. To elucidate the underlying mechanism of action, molecular docking studies were conducted to analyze ligand-target interactions at the atomic level, revealing favorable binding conformations within key regulatory proteins implicated in oncogenesis. Furthermore, enzyme kinetics and dose-response inhibition assays were performed to characterize the interaction dynamics and establish potential modes of inhibition. The ADMET profile of the lead compounds was also evaluated in silico, supporting their drug-likeness and safety for further preclinical development. These findings contribute valuable scaffolds for the development of new anticancer agents and highlight the importance of integrating chemical synthesis with molecular biology techniques in the discovery of targeted cancer therapeutics.
Keywords: ADMET; Cancer; Cell proliferation; Enzyme kinetics; Immunotherapy; Molecular Docking; Synthesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures














References
-
- Garza-Ortiz, A. et al. A new family of Ru (II) complexes with a tridentate pyridine Schiff-base ligand and bidentate coligands: Synthesis, characterization, structure and in vitro cytotoxicity studies. New. J. Chem.37 (11), 3450–3460 (2013). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

