Multiparity induces persistent myocardial structural, functional and transcriptomic remodelling in mice
- PMID: 40624174
- PMCID: PMC12234663
- DOI: 10.1038/s41598-025-08248-z
Multiparity induces persistent myocardial structural, functional and transcriptomic remodelling in mice
Abstract
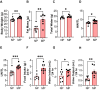
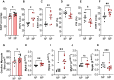
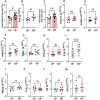
Pregnancy poses unique hemodynamic and metabolic demands on the heart. Recent data suggests that a history of multiple pregnancies may be associated with a late-term increased risk of heart failure with preserved ejection fraction (HFpEF), however, the mechanistic basis is unknown. We postulated that the repetitive biological and hemodynamic impact of multiple pregnancies could cause persistent myocardial remodelling providing a substrate for subsequent HFpEF. Multiparous (MP) mice (C57Bl/6J) aged 24 months were compared to age-matched non-parous (NP) mice (n = 8) in a blinded analysis. We evaluated blood pressure, body composition, gross weight and histologic cardiac structure. Cardiac function was assessed by echocardiography together with transcriptomic analysis of the left ventricular myocardium. At 15 months post oestrous cycle disruption, MP mice continued to demonstrate a constellation of myocardial alterations including higher heart to tibial length ratio (P < 0.05), body weight (P < 0.01), and fat mass (P < 0.01) compared to NP mice. MP mice had increased isovolumetric relaxation time (P < 0.01), increased end-diastolic (P < 0.05) and systolic volumes (P < 0.005), and mildly reduced left ventricular ejection fractions (LVEF) (P < 0.005) compared to non-parous mice. This was accompanied by marked elevations in mRNA levels of cardiac markers; Myh6 (P < 0.01) and Nppa (P < 0.01). mRNA levels of Il18 (P < 0.05) and the basal extracellular matrix protein-fibronectin (P < 0.05) were also increased, with a significant increase in interstitial fibrosis (P < 0.05). Bulk RNA sequencing (RNA Seq) further revealed persistent differential expression of 128 genes with over/under-represented pathways involved in extracellular matrix (ECM) regulation and organic anion/ion transport, respectively. Multiparity alters cardiac gene profile and causes myocardial remodelling that persists into later life, providing a potential foundation for the HF phenotypes such as HFpEF. Further studies are required to investigate the nature of the interaction between pregnancy-associated remodelling and other factors known to be associated with HFpEF development.
Keywords: Cardiac remodelling; Fibrosis; HFpEF; Hypertrophy; Multiparity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Sex-specific cardiometabolic multimorbidity, metabolic syndrome and left ventricular function in heart failure with preserved ejection fraction in the UK Biobank.Cardiovasc Diabetol. 2025 Jun 4;24(1):238. doi: 10.1186/s12933-025-02788-4. Cardiovasc Diabetol. 2025. PMID: 40468351 Free PMC article.
-
Computational investigation of the role of ventricular remodelling in HFpEF: The key to phenotype dissection.Comput Biol Med. 2024 Sep;180:109019. doi: 10.1016/j.compbiomed.2024.109019. Epub 2024 Aug 16. Comput Biol Med. 2024. PMID: 39153393
-
Beta-blockers in patients without heart failure after myocardial infarction.Cochrane Database Syst Rev. 2021 Nov 5;11(11):CD012565. doi: 10.1002/14651858.CD012565.pub2. Cochrane Database Syst Rev. 2021. PMID: 34739733 Free PMC article.
-
An ovary-intact postmenopausal HFpEF mouse model; menopause is more than just estrogen deficiency.Am J Physiol Heart Circ Physiol. 2025 Apr 1;328(4):H719-H733. doi: 10.1152/ajpheart.00575.2024. Epub 2025 Feb 18. Am J Physiol Heart Circ Physiol. 2025. PMID: 39963865 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med.385(16), 1451–1461 (2021). - PubMed
-
- Solomon, S. D. et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med.387(12), 1089–1098 (2022). - PubMed
-
- Shah, S. J. et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): A randomised, multicentre, blinded, sham-controlled trial. Lancet399(10330), 1130–1140 (2022). - PubMed
-
- Owan, T. E. et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med.355(3), 251–259 (2006). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

