PD-L1 expression and its association with clinicopathological and computed tomography features in surgically resected non-small cell lung cancer: a retrospective cohort study
- PMID: 40624192
- PMCID: PMC12234839
- DOI: 10.1038/s41598-025-10437-9
PD-L1 expression and its association with clinicopathological and computed tomography features in surgically resected non-small cell lung cancer: a retrospective cohort study
Abstract
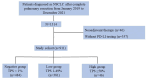
Although some studies have assessed the correlation between clinicopathological features and programmed death-ligand 1 (PD-L1) in patients with non-smallcell lung cancer (NSCLC), few studies have focused on computed tomography (CT) signs. The results of some previous studies are inconsistent and contradictory. Therefore, this study aimed to analyze the clinicopathological and CT features of NSCLC patients with different PD-L1 expression levels. This retrospective analysis included 911 NSCLC patients who undergone pulmonary resection, and most of them were early-stage. PD-L1 expression was assessed by immunohistochemistry with the Dako PD-L1 22C3 pharmDx kit. Clinicopathological features and CT signs were investigated according to different PD-L1 expression levels. The prevalence of PD-L1 expression in the resected NSCLC patients was 46.9% (427/911), with 381 patients low expression (41.8%) and 46 patients high expression (5%). Male sex, current smoking status, higher positron emission tomography-computed tomography (PET-CT) SUVmax, and elevated serum carcinoembryonic antigen levels were more frequently observed in patients with high PD-L1 expression. The frequencies of squamous cell carcinoma type, spread through air space (STAS), TP53 mutations, advanced pathological stages, and micropapillary/solid subtype were significantly higher in patients with PD-L1 positive tumors (TPS ≥ 1%) than in those with PD-L1 negative tumors (TPS < 1%). ALK rearrangement was higher in patients with low-expression, and PD-L1-positive patients had bigger tumor diameter. The proportion of solid nodules and consolidation to tumor were higher in high-expression than those in the low- or negative expression PD-L1 patients. Regarding structural characteristic features, there were no differences in the frequency of irregular borders, pleural retraction, air bronchograms, bubble-like lucency or cavities, and vascular convergence among the three groups. The frequency of lobulated margins and spiculation was significantly higher in patients with PD-L1 positive tumors than in those with PD-L1 negative tumors. Patients with PD-L1 expression in NSCLC often exhibit certain clinicopathological characteristics. CT features may not reliably correlate with PD-L1 expression across different stages of lung cancer. Immunohistochemistry (IHC), using relevant kits, remains essential for further evaluation of PD-L1 expression levels.
Keywords: Clinicopathology; Computed tomography; Genomic mutation; Non-small cell lung cancer; Programmed death-ligand 1.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study was performed in accordance with the guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki, and was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital(S2024-351-01). The requirement for individual informed consent was waived by the ethics committee of the PLA General Hospital, as this was a retrospective study.
Figures


Similar articles
-
Clinicopathological and molecular characteristics associated with PD-L1 expression in non-small cell lung cancer: a large-scale, multi-center, real-world study in China.J Cancer Res Clin Oncol. 2021 May;147(5):1547-1556. doi: 10.1007/s00432-020-03444-y. Epub 2020 Nov 16. J Cancer Res Clin Oncol. 2021. PMID: 33196892 Free PMC article.
-
Alterations and correlation between DNA damage and repair response and PD-L1 expression in non-small cell lung cancers.BMC Cancer. 2025 Jul 17;25(1):1183. doi: 10.1186/s12885-025-14065-4. BMC Cancer. 2025. PMID: 40676600 Free PMC article.
-
PD-L1 Expression and Comprehensive Genomic Profiling in Advanced NSCLC: A Single-Centre Experience.Int J Mol Sci. 2025 Jul 1;26(13):6348. doi: 10.3390/ijms26136348. Int J Mol Sci. 2025. PMID: 40650126 Free PMC article.
-
PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review.Lung Cancer. 2017 Oct;112:200-215. doi: 10.1016/j.lungcan.2017.08.005. Epub 2017 Aug 10. Lung Cancer. 2017. PMID: 29191596
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
References
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin.72 (1), 7–33 (2022). - PubMed
-
- Felip, E. et al. IMpower010 investigators. Adjuvant Atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet9 (10308), 1344–1357 (2021). - PubMed
-
- O’Brien, M. et al. EORTC-1416-LCG/ETOP 8–15 – PEARLS/KEYNOTE-091 investigators. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol.23 (10), 1274–1286 (2022). - PubMed
-
- Mok, T. S. K. et al. KEYNOTE-042 investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet393 (10183), 1819–1830 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

