Association between dietary copper intake and cognitive function in American older adults: NHANES 2011-2014
- PMID: 40624281
- PMCID: PMC12234787
- DOI: 10.1038/s41598-025-09280-9
Association between dietary copper intake and cognitive function in American older adults: NHANES 2011-2014
Abstract
This cross-sectional observational study examined the association between dietary copper intake and cognitive function in American older adults, using data from the 2011 to 2014 National Health and Nutrition Examination Survey (NHANES). Analyzing a total of 2420 participants, dietary copper intake was determined by averaging two 24-h dietary recalls, whereas cognitive function was assessed by the Digit Symbol Substitution Test (DSST), the Animal Fluency Test (AFT), a Consortium to Establish a Registry for Alzheimer's disease (CERAD) subtest and global cognition Z score. Multivariate linear regression models were used to explore the association between copper levels and cognitive function. Higher copper intake was associated with higher cognitive scores. In the fully adjusted model, compared to the lowest quartile (Q1), the highest quartile (Q4) of copper intake was associated with related to higher cognitive scores (DSST: β = 3.80, 95% CI 1.90,5.70; AFT: β = 1.23, 95% CI 0.48,1.99; CERAD-IRT: β = 0.58, 95% CI - 0.06,1.22; CERAD-DRT: β = 0.47, 95% CI 0.15,0.80; Z score: β = 0.20, 95% CI 0.10,0.29), particularly in participants with a history of stroke. Multivariate smooth spline analysis revealed that dietary copper intake was related to DSST, AFT and Z score in an inverted L-shaped nonlinear manner. The inflection point of copper was 1.63 mg/day for DSST, 1.42 mg/day for AFT and 1.22 mg/day for the Z score. Further longitudinal research is necessary to substantiate these findings.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: The National Health and Nutrition Examination Survey (NHANES) is conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. All participants signed written informed consent. Consent for publication: The authors have no ethical, legal, and financial conflicts related to the article. All authors read and approved the manuscript for publication.
Figures
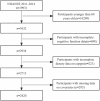
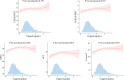

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

