The clinical application value of the KU-F40 fully automatic fecal analyzer for the detection of fecal parasites: A large-sample retrospective study
- PMID: 40624366
- PMCID: PMC12234752
- DOI: 10.1038/s41598-025-09935-7
The clinical application value of the KU-F40 fully automatic fecal analyzer for the detection of fecal parasites: A large-sample retrospective study
Abstract
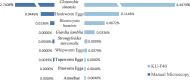
Improving the sensitivity of parasite detection is an urgent task for global public health. The traditional manual microscopy method still has some shortcomings in fecal parasite detection. This study aimed to evaluate the clinical application value of the KU-F40 fully automated fecal analyzer in fecal parasite detection by comparing the fecal detection results of the manual microscopy method with those of the KU-F40 instrumental method. Fecal test results were collected from January to June 2023 and January to June 2024, and divided into manual microscopy group (n = 51,627) and KU-F40 instrumental group (n = 50,606) according to methodology. The detection levels and species of parasites detected by the two methods were retrospectively analyzed. The KU-F40 instrumental group had a higher parasite detection level (8.74%) than the manual microscopy group (2.81%), with statistically significant differences between groups (χ2 = 1661.333, P < 0.05). Five species of parasites were detected by the manual microscopy method and nine species of parasites were detected by the KU-F40 instrumental method, of which the detection levels of Clonorchis sinensis eggs, hookworm eggs and Blastocystis hominis were higher than those of the manual microscopy method, with statistically significant differences (P < 0.05). Although the detection levels of tapeworm eggs and Strongyloides stercoralis were also higher than the manual microscopy method, the differences were not statistically significant (P > 0.05). The KU-F40 fully automatic fecal analyzer has a higher sensitivity to parasite detection and holds significant clinical application value in the detection of fecal parasites. With the combination of manual re-examination, the accuracy of test results can be significantly improved.
Keywords: Clinical application value; Fecal detection; KU-F40 fully automatic fecal analyzer; Manual microscopy; Parasite.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical standards: The research protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval Number: 2024-E0831). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent: Informed consent was obtained from all subjects or their legal guardians.
Figures
References
-
- Wenhua, G. & Tingting, Y. Research progress on clinical laboratory examination methods for intestinal parasites. Trace Elem. Health Res.41(5), 83–86 (2024).
-
- Zehong, Q. & Yanqin, W. Comparison of the application of automatic stool analyzer and manual microscopy in fecal specimen detection. Smart Health.8(35), 20–23 (2022).
-
- Hong, S., Yusan, W. & Ziyu, S. National Clinical Laboratory Testing Procedures. 4th Edition. Beijing: People’s Health Publishing House pp.175–177 (2015).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

