AKT and DUBs: a bidirectional relationship
- PMID: 40624457
- PMCID: PMC12232702
- DOI: 10.1186/s11658-025-00753-3
AKT and DUBs: a bidirectional relationship
Abstract
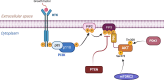
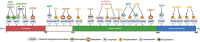
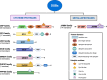
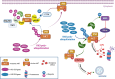
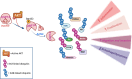
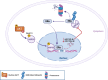
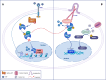
The serine/threonine kinase Akt is crucial for cell physiology and can also contribute to pathology if its activation and regulation is disturbed. This kinase phosphorylates several substrates involved in mechanisms that are altered in human disease. AKT is regulated by several post-translational modifications (PTMs), including ubiquitination/deubiquitination. Ubiquitination can both target AKT to the proteasome and promote its activation. The interplay with the deubiquitination mechanism plays a crucial role in almost all biological activities of AKT. Information on the mechanisms of AKT deubiquitination and its key players has evolved rapidly in recent years along with the development of potential targeting strategies, although many of them are still unclear. Nevertheless, AKT in turn regulates various deubiquitinases (DUBs), suggesting further targeting strategies for human diseases. In this review, we aim to provide an up-to-date overview of the dual relationship between AKT and DUBs with respect to potential translational aim.
Keywords: AKT kinase; Deubiquitinases; Phosphorylation; Post-translational modifications.
© 2025. The Author(s).
Figures

Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Deubiquitinating enzymes: potential regulators of the tumor microenvironment and implications for immune evasion.Cell Commun Signal. 2024 May 7;22(1):259. doi: 10.1186/s12964-024-01633-7. Cell Commun Signal. 2024. PMID: 38715050 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The Role of Deubiquitinating Enzymes in Primary Bone Cancer.Mol Biotechnol. 2025 Aug;67(8):3027-3040. doi: 10.1007/s12033-024-01254-y. Epub 2024 Aug 23. Mol Biotechnol. 2025. PMID: 39177860 Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/Pkbalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in MICE. J Biol Chem. 2001;276(42):38349–52. 10.1074/jbc.C100462200. - PubMed
-
- Eijkelenboom A, Burgering BM. Foxos: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83–97. 10.1038/nrm3507. - PubMed
-
- Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor P27(Kip1) by Pkb/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8(10):1136–44. 10.1038/nm762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

