SMPDL3B a novel biomarker and therapeutic target in myalgic encephalomyelitis
- PMID: 40624584
- PMCID: PMC12236014
- DOI: 10.1186/s12967-025-06829-0
SMPDL3B a novel biomarker and therapeutic target in myalgic encephalomyelitis
Erratum in
-
Correction: SMPDL3B a novel biomarker and therapeutic target in myalgic encephalomyelitis.J Transl Med. 2025 Aug 14;23(1):911. doi: 10.1186/s12967-025-06900-w. J Transl Med. 2025. PMID: 40813669 Free PMC article. No abstract available.
Abstract
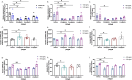
Background: Sphingomyelin phosphodiesterase acid-like 3B (SMPDL3B) is emerging as a potential biomarker and therapeutic target in myalgic encephalomyelitis (ME), a complex multisystem disorder characterized by immune dysfunction, metabolic disturbances, and persistent fatigue. This study investigates the role of SMPDL3B in ME pathophysiology and explores its clinical relevance.
Methods: A case-control study was conducted in two independent cohorts: a Canadian cohort (249 ME patients, 63 controls) and a Norwegian replication cohort (141 ME patients). Plasma and membrane-bound SMPDL3B levels were quantified using ELISA and flow cytometry. Gene expression of SMPDL3B and PLCXD1, encoding phosphatidylinositol-specific phospholipase C (PI-PLC), was analyzed by qPCR. The effects of dipeptidyl peptidase-4 (DPP-4) inhibitors-vildagliptin, saxagliptin, and linagliptin-on modulation of membrane-bound and soluble SMPDL3B were assessed in vitro by qPCR, flow cytometry and ELISA.
Results: ME patients exhibited significantly elevated plasma SMPDL3B levels, which correlated with symptom severity. Flow cytometry revealed a reduction in membrane-bound SMPDL3B in monocytes, accompanied by increased PLCXD1 expression and elevated plasma levels of PI-PLC and SMPDL3B. These findings suggest that immune dysregulation in ME may be linked to enhanced cleavage of membrane-bound SMPDL3B by PI-PLC. Sex-specific differences were observed, with female ME patients displaying higher plasma SMPDL3B levels, an effect influenced by estrogen. In vitro, estradiol upregulated SMPDL3B expression, indicating hormonal regulation. Vildagliptin and saxagliptin were tested for their potential to inhibit PI-PLC activity independently of their role as DPP-4 inhibitors, and restored membrane-bound SMPDL3B while reduced its soluble form.
Conclusions: SMPDL3B emerges as a key biomarker for ME severity and immune dysregulation, with its activity influenced by hormonal and PI-PLC regulation. The ability of vildagliptin and saxagliptin to preserve membrane-bound SMPDL3B and reduce its soluble form via PI-PLC inhibition suggests a novel therapeutic strategy. These findings warrant clinical trials to evaluate their potential in mitigating immune dysfunction and symptom burden in ME.
Keywords: Myalgic encephalomyelitis (ME); Phosphatidylinositol-specific phospholipase C (PI-PLC); Saxagliptin; Sphingomyelin phosphodiesterase acid-like 3b (SMPDL3B); Vildagliptin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of CHU Sainte-Justine (protocol #4047). Informed consent was obtained from all participants involved in the study. Consent for publication: Not applicable. Competing interests: Pr. Moreau is Director of Interdisciplinary Canadian Collaborative Myalgic Encephalomyelitis (ICanCME) Research Network, a national research network funded by The Canadian Institutes of Health Research (Grant MNC—166142 to Pr. Moreau). Pr. Moreau, Dr. Fluge and Pr. Mella are members of the Scientific Advisory Board of the Open Medicine Foundation (USA). The authors declare no other competing interests.
Figures




References
-
- Canada OMF. Qu’est-ce que l’EM 2022. Available from: https://www.omfcanada.ngo/quest-ce-que-lem-sfc/?lang=fr.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

