Immunoglobulin A/PIGR axis as potential mediators of human abdominal aortic aneurysms revealed by topologically resolved proteomics
- PMID: 40624587
- PMCID: PMC12232777
- DOI: 10.1186/s12967-025-06758-y
Immunoglobulin A/PIGR axis as potential mediators of human abdominal aortic aneurysms revealed by topologically resolved proteomics
Abstract
Background: Abdominal aortic aneurysm (AAA) is an asymptomatic chronic disease of the aorta and its evolution is unpredictable. Despite the existence of several pathological mechanisms contributing to the dilation of the human AAA wall, there is currently no specific therapy to prevent the fatal rupture of the aorta. Our objective was to identify novel mediators and/or biomarkers involved in the instability of the aortic wall that could help to prevent AAA progression.
Methods: Multiplexed quantitative proteomic analysis of human AAA and healthy aortic wall (medial and adventitial layers) was performed. Results were subsequently validated by western blot and immunohistochemistry, as well as by turbidimetry/ELISA of tissue-conditioned media. In addition, immunoglobulins A1 and A2 (IGA1 and IGA2) plasma levels were analyzed by turbidimetry in a pilot study [controls (n = 22) and AAA patients (n = 22)] and in a validation study with a 6-year follow-up [controls (n = 64) and AAA patients (n = 189)]. In vitro experiments were performed in THP-1-derived macrophages (basal or polarized to M1 or M2). Polymeric immunoglobulin receptor (PIGR) mRNA expression and secretion in macrophages were analyzed by Q-PCR and ELISA, respectively. Finally, the hematopoietic contribution of PIGR was assessed in experimental AAA (Ldlr-/- mice fed an atherogenic diet and 1 μg/Kg/min angiotensin II infusion for 28 days) by bone marrow transplantation experiments.
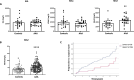
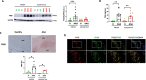
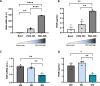
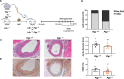
Results: Functional analysis of biological pathways altered in human AAA wall revealed a significant upregulation of components of the adaptive immune response, including IGHA1 and IGHA2, as well as the IGA receptor, PIGR. In addition, IGA2, but not IGA1, plasma levels were significantly increased in a pilot study of AAA patients relative to controls (489 ± 38 vs 344 ± 36 mg/L, p < 0.01). This finding was further validated in a larger cohort, confirming the association of IGA2 with AAA presence independent of risk factors and treatments [OR = 2.140 (1.109-4.130), P < 0.05]. Furthermore, in the validation cohort, elevated IGA2 plasma levels were independently associated with AAA progression [HR = 1.941 (1.108-3.399), p < 0.05]. PIGR colocalized with macrophages in the AAA wall and, PIGR mRNA levels were increased following the differentiation of THP-1 monocytes into macrophages, as well as in M1-polarized THP-1 macrophages compared to M2 macrophages. Pigr deficiency in hematopoietic cells resulted in a significantly reduced AAA incidence (14 vs 57%) and decreased macrophage infiltration (3.5 ± 0.5 vs 5.6 ± 0.7%).
Conclusions: Increased IGA and PIGR is observed in the AAA wall. Pigr deficiency in hematopoietic cells decreases AAA progression, suggesting a therapeutic role for PIGR in AAA.
Keywords: Abdominal aortic aneurysm; Biomarkers; Immune response; Immunoglobulin A; Polymeric immunoglobulin receptor; Proteomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol of this study was approved by the Ethics Committee of IIS-FUNDACIÓN JIMÉNEZ DÍAZ (PIC140-20_FJD), as well as the corresponding Ethics Committees of France and Denmark as specified in the methods sections. All the participating patients provided written informed consent. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Exploring circulating cell-free DNA as a biomarker and as an inducer of AIM2-inflammasome-mediated inflammation in patients with abdominal aortic aneurysm.Sci Rep. 2025 Jun 20;15(1):20196. doi: 10.1038/s41598-025-06220-5. Sci Rep. 2025. PMID: 40542152 Free PMC article.
-
Laparoscopic surgery for elective abdominal aortic aneurysm repair.Cochrane Database Syst Rev. 2017 May 4;5(5):CD012302. doi: 10.1002/14651858.CD012302.pub2. Cochrane Database Syst Rev. 2017. PMID: 28471523 Free PMC article.
-
The role of polymeric immunoglobulin receptor in inflammation-induced tumor metastasis of human hepatocellular carcinoma.J Natl Cancer Inst. 2011 Nov 16;103(22):1696-712. doi: 10.1093/jnci/djr360. Epub 2011 Oct 24. J Natl Cancer Inst. 2011. PMID: 22025622 Free PMC article.
-
Medical treatment for small abdominal aortic aneurysms.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009536. doi: 10.1002/14651858.CD009536.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972146 Free PMC article.
-
Deciphering the transcriptomic characteristic of lactate metabolism and the immune infiltration landscape in abdominal aortic aneurysm.Biochem Biophys Res Commun. 2025 Aug 30;776:152198. doi: 10.1016/j.bbrc.2025.152198. Epub 2025 Jun 14. Biochem Biophys Res Commun. 2025. PMID: 40532306
References
-
- Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for abdominal aortic aneurysm: US preventive services task force recommendation statement. JAMA. 2019;322(22):2211–8. - PubMed
-
- Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16(4):225–42. - PubMed
-
- Torres-Fonseca M, Galan M, Martinez-Lopez D, Cañes L, Roldan-Montero R, Alonso J, et al. Pathophisiology of abdominal aortic aneurysm: biomarkers and novel therapeutic targets. Clinica e investigacion en arteriosclerosis : publicacion oficial de la Sociedad Espanola de Arteriosclerosis. 2019;31(4):166–77. - PubMed
-
- Li B, Khan H, Shaikh F, Zamzam A, Abdin R, Qadura M. Identification and evaluation of blood-based biomarkers for abdominal aortic aneurysm. J Proteome Res. 2024;23(6):2279–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

