Immunoelectron microscopy and biochemical studies using three anti-tau antibodies in human brains: associations between pTau and ribosomes
- PMID: 40624595
- PMCID: PMC12232755
- DOI: 10.1186/s40478-025-02072-2
Immunoelectron microscopy and biochemical studies using three anti-tau antibodies in human brains: associations between pTau and ribosomes
Abstract
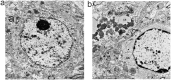
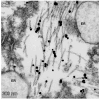
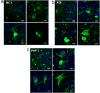
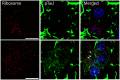
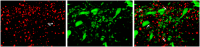
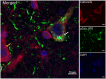
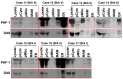
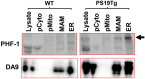
The hallmark neuropathological lesions of Alzheimer's disease (AD) are extracellular amyloid-beta (Aβ) plaque deposits and intracellular Tau neurofibrillary tangles (NFTs). Identifying the intracellular localization of early pathologic changes can enhance our understanding of disease mechanisms and stimulate new approaches in diagnosis and treatment. Despite extensive biochemical studies of AD-related protein aggregates, there have been relatively few studies recently in terms of transmission electron microscopy of proteinaceous lesions in human brains across a range of disease severity. Here we performed immunoelectron microscope studies used three anti-Tau antibodies (MC-1, AT8, and PHF-1) on short post-mortem interval (PMI) human brain tissues obtained from the University of Kentucky Alzheimer's Disease Research Center (UK-ADRC) autopsy cohort, along with corresponding biochemical and immunofluorescent studies. Although these three antibodies have been reported to label different phases of NFT formation, in our hands they all tended to stain pathologic structures along a continuum that included pretangles and mature NFTs. Immunoelectron microscopy studies, augmented by serial sectioning, revealed that all three Tau antibodies recognize both granular and fibrillary structures in pretangles and early-stage NFTs. Phosphorylated Tau (pTau) immunoreactivity often exhibited a peri-nuclear distribution. The pTau was frequently found localized to ribosomes, either free within the cytoplasm or attached to the endoplasmic reticulum (ER). This observation aligns with previous descriptions, but the enhanced ultrastructural preservation provided improved resolution. Subcellular fractionation and Western blot analyses confirmed the enrichment of pTau in the ER fractions in AD brains. Interestingly, total Tau (including non-phosphorylated Tau) did not preferentially co-purify with the ER in non-AD brains. Immunofluorescent staining revealed that pTau immunoreactivity evolved from cytoplasmic granules in pretangles, with an intracytoplasmic distribution that overlapped complementarily with ribosome and ER markers. Our results suggest that biochemical associations between pTau with ribosomes and ER are a common phenomenon in human aged brains.
Keywords: Alzheimer’s disease; Endoplasmic reticulum; Human brain; ImmunoEM; Ribosome; Serial sections; Subcellular fractionation; Tau phosphorylation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Procedures and protocols related to the human brain autopsies were conducted with consent and approved by the University of Kentucky Institutional Review Board (IRB no. 44009). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures












References
-
- Abisambra JF, Jinwal UK, Blair LJ, O’Leary JC 3rd, Li Q, Brady S, Wang L, Guidi CE, Zhang B, Nordhues BAet al et al (2013) Tau accumulation activates the unfolded protein response by impairing Endoplasmic reticulum-associated degradation. J Neurosci 33:9498–9507. 10.1523/JNEUROSCI.5397-12.2013 - PMC - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT (2002) Specific Tau phosphorylation sites correlate with severity of neuronal cytopathology in alzheimer’s disease. Acta Neuropathol 103:26–35. 10.1007/s004010100423 - PubMed
-
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM (1989) Accumulation of abnormally phosphorylated Tau precedes the formation of neurofibrillary tangles in alzheimer’s disease. Brain Res 477:90–99. 10.1016/0006-8993(89)91396-6 - PubMed
-
- Barnes DM (1985) Alz 50 recognizes an alzheimer’s protein. Sci (New York NY) 230:1260. 10.1126/science.4071048 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

