Cardiac myosin inhibitors in hypertrophic cardiomyopathy
- PMID: 40624601
- PMCID: PMC12232738
- DOI: 10.1186/s44348-025-00052-7
Cardiac myosin inhibitors in hypertrophic cardiomyopathy
Abstract
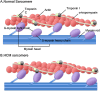
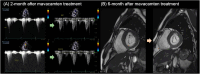
Mavacamten, the first selective and reversible cardiac myosin inhibitor (CMI), has been introduced to the clinical arena for the treatment of obstructive hypertrophic cardiomyopathy (HCM). By reducing excessive actin-myosin cross-bridging, this agent decreases myocardial contractility and alleviates the dynamic left ventricular outflow tract (LVOT) obstruction in obstructive HCM. In the EXPLORER-HCM trial, mavacamten significantly improved exercise capacity, symptoms, and LVOT pressure gradients, while the VALOR-HCM trial proved it can obviate the need for septal reduction therapy in patients who were deemed to be candidates for septal reduction therapy. Notably, long-term data (MAVA-LTE study) has demonstrated sustained benefits up to 180 weeks, with < 10% experiencing transient reductions in left ventricular ejection fraction < 50% and only 1.3% of permanent discontinuation rate. Aficamten, a next-generation CMI with a shorter half-life, has also demonstrated comparable efficacy. Reverse remodeling following treatment was noted in both agents. In nonobstructive HCM, preliminary studies (MAVERICK-HCM trial and cohort 4 of REDWOOD-HCM trial) have reported improvements in cardiac serum biomarkers and symptoms. However, the preliminary results from phase 3 trials (ODYSSEY-HCM trial) revealed that primary endpoints were not met in nonobstructive HCM. Regarding safety, both were generally well tolerated. Although an LVEF reduction occurred in some patients, it was reversible with a dose reduction or a short-term drug cessation. These results emphasize careful dosing strategy with regular echocardiographic monitoring. Real-world data have also demonstrated consistent efficacy and safety across varying ethnic groups without new safety signals. CMI is a major advance in HCM management. However, future studies must provide data on hard clinical outcomes, such as heart failure hospitalization or death. Ongoing trials comparing CMI to traditional first-line therapies, such as β-blockers, will clarify their potential role as an initial therapeutic option.
Keywords: Cardiac myosin; Cardiac myosin inhibitor; Cardiomyopathy, hypertrophic; Diastolic dysfunction; Review; Therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This review article does not contain any studies with human participants and thus does not require approval from our institutional review board. Consent for publication: Not applicable. Competing interests: H.-K.K. is a consultant of BMS.
Figures

References
-
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Circulation. 1995;92:785–9. - PubMed
-
- Braunwald E, Lambrew CT, Rockoff SD, Ross J, Morrow AG. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation. 1964;30:3–119. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

