Quercetin nanoformulation-embedded hydrogel inhibits osteopontin mediated ferroptosis for intervertebral disc degeneration alleviation
- PMID: 40624664
- PMCID: PMC12235785
- DOI: 10.1186/s12951-025-03574-w
Quercetin nanoformulation-embedded hydrogel inhibits osteopontin mediated ferroptosis for intervertebral disc degeneration alleviation
Abstract
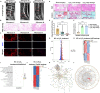
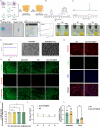
Reactive oxygen species (ROS) play a pivotal role in multiple events during the progression of intervertebral disc degeneration (IDD). Hence, the precision treatment targets associated with ROS should be further explored to promote developing effective therapeutic strategies. In this study, by analyzing specimens from patients and RNA sequencing of ROS-induced human primary nucleus pulposus cells (NPCs), osteopontin (OPN) and ferroptosis were identified as critical molecular entities and cellular pathways implicated in ROS-mediated IDD. Subsequent animal models and cellular assays determined that ROS induced upregulation of OPN, which in turn triggered ferroptosis in NPCs and intervertebral discs, consequently leading to IDD. Building upon these findings, a comprehensive screening of molecular drug database revealed that quercetin, an antioxidant molecule compound, possesses the capacity to couple OPN, thereby mitigating OPN-induced ferroptosis and IDD. In addition, the compound of quercetin for targeting OPN was encapsulated in phenylboric acid modified dendrimer (G3-PBA) nanoparticles to improve its solubility, and then embedded in a ROS-degradable and injectable hydrogel, thereby achieving on-demand release of quercetin with the progression of IDD. Collectively, this study not only identified a novel therapeutic target, but also engineered an effective therapeutic strategy intended for the autonomous management of IDD.
Keywords: Ferroptosis; Injectable hydrogel; Intervertebral disc degeneration; Osteopontin; Quercetin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine (Phila Pa 1976). 2004;29(23):2679–90. - PubMed
-
- Teraguchi M, Yoshimura N, Hashizume H, Yamada H, Oka H, Minamide A, Nagata K, Ishimoto Y, Kagotani R, Kawaguchi H, et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: the Wakayama spine study. Osteoarthritis Cartilage. 2017;25(7):1122–31. - PubMed
MeSH terms
Substances
Grants and funding
- 2023YFC2508806/National Key Research and Development Program of China
- 82474554/National Natural Science fund of China
- 231111310500/Key Research and Development Project in Henan Province
- ZYYC2023ZD/Scientific Research Project of Henan Zhongyuan Medical Science and Technology Innovation and Development Foundation
- 20214Y0132/the Shanghai Bureau of Health
LinkOut - more resources
Full Text Sources
Research Materials

