CDCP1 promotes the malignant phenotypes of nasopharyngeal carcinoma via the Wnt/β-catenin signaling pathway
- PMID: 40624715
- PMCID: PMC12235957
- DOI: 10.1186/s12896-025-01001-4
CDCP1 promotes the malignant phenotypes of nasopharyngeal carcinoma via the Wnt/β-catenin signaling pathway
Abstract
Background: CUB domain-containing protein 1 (CDCP1), a type I transmembrane glycoprotein, is abundantly expressed in various cancers. However, its role and mechanism in nasopharyngeal carcinoma (NPC) remain ambiguous.
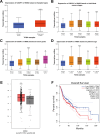
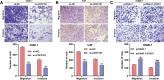
Methods: The UALCAN and GEPIA databases were analyzed to explore CDCP1 expression and survival prognosis in head and neck squamous cell carcinoma (HNSC) patients. Fifteen pairs of NPC tissues and adjacent normal tissues were collected for CDCP1 expression analysis. CCK-8 assays, flow cytometry, and transwell assays were performed on NPC cell lines (C666-1, 5-8 F, and HONE-1). The impact of GSK-3β inhibitor LiCl on C666-1 cells after CDCP1 knockdown was investigated. A C666-1 xenograft model was established for in vivo validation.
Results: CDCP1 was overexpressed in HNSC patients, and elevated CDCP1 correlated with poor survival. NPC tissues confirmed CDCP1 upregulation compared to normal tissues. CDCP1 knockdown in C666-1 and 5-8 F cells inhibited proliferation, migration, invasion, and promoted apoptosis, while LiCl partially reversed these effects. In vivo, CDCP1 silencing suppressed tumor growth, downregulated PCNA, Wnt3a, β-catenin, and p-GSK-3β, and upregulated cleaved caspase-3 and E-cadherin. CDCP1 overexpression in HONE-1 cells produced opposing effects.
Conclusions: In summary, CDCP1 promotes NPC progression via the Wnt/β-catenin pathway, suggesting its potential as a therapeutic target.
Clinical trial number: Not applicable.
Keywords: CDCP1; Nasopharyngeal carcinoma; Wnt/β-catenin signaling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The samples utilized in this study were approved by the Medical Ethics Committee of the, Nanyang Central Hospital (Ethical Number: 202401007, Henan, China). Informed written consent was collected from all participants in accordance with the Declaration of Helsinki. The animal research was conducted in accordance with the National Institutes of Health Guidelines and was approved by the Animal Care and Use Committee of the Nanyang Institute of Technology (Henan, China). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Effect of MicroRNA-4713-3p on Promoting Malignant Progression of Nasopharyngeal Carcinoma via Targeted Inhibition of EPHX3 to Activate Wnt/β-Catenin Signaling Pathway.J Biochem Mol Toxicol. 2025 Sep;39(9):e70450. doi: 10.1002/jbt.70450. J Biochem Mol Toxicol. 2025. PMID: 40878263
-
Role of the CEACAM7-JAK2/STAT3-BST2 Axis in the migration of nasopharyngeal carcinoma cells.Biochim Biophys Acta Mol Basis Dis. 2025 Oct;1871(7):167948. doi: 10.1016/j.bbadis.2025.167948. Epub 2025 Jun 5. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40482926
-
CUB domain-containing protein 1 signaling dysregulates gemcitabine metabolism contributing to therapeutic resistance in T24 cells.PLoS One. 2025 Sep 2;20(9):e0331289. doi: 10.1371/journal.pone.0331289. eCollection 2025. PLoS One. 2025. PMID: 40892739 Free PMC article.
-
Targeting GSK-3β for adipose dysfunction and cardiovascular complications of metabolic disease: An entangled WNT/β-catenin question.FASEB J. 2024 Dec 13;38(24):e70273. doi: 10.1096/fj.202402470R. FASEB J. 2024. PMID: 39726401 Review.
-
Balancing Immunity: GSK-3's Divergent Roles in Dendritic Cell-Mediated T-Cell Priming and Memory Responses.Int J Mol Sci. 2025 Jun 25;26(13):6078. doi: 10.3390/ijms26136078. Int J Mol Sci. 2025. PMID: 40649856 Free PMC article. Review.
References
-
- Parlak S, Yazici G, Dolgun A, Ozgen B. The evolution of bone marrow signal changes at the skull base in nasopharyngeal carcinoma patients treated with radiation therapy. Radiol Med. 2021;126(6):818–26. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

