Development and preclinical characterization of AMG 329: a human antibody neutralizing FLT3 ligand
- PMID: 40624796
- PMCID: PMC12239813
- DOI: 10.1080/19420862.2025.2527677
Development and preclinical characterization of AMG 329: a human antibody neutralizing FLT3 ligand
Abstract
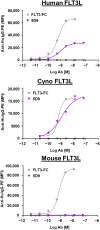
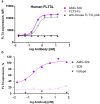
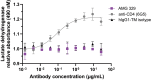
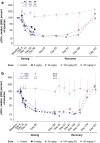
The feline McDonough sarcoma-like tyrosine kinase 3 (FLT3)/FLT3 ligand (FLT3L) signaling pathway regulates the development and activity of dendritic cells (DCs) and other myeloid cells, including monocytes. FLT3L, DCs, and monocytes have been implicated in several autoimmune diseases. Here, we describe the development and characterization of a human immunoglobulin G1λ monoclonal antibody (AMG 329; formerly MEDI1116/VIB-1116/HZN-1116) targeting human FLT3L. AMG 329 was derived from a large human combined antibody display library; it was optimized to enhance affinity for FLT3L and reduce antibody dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity. Binding affinity was determined by surface plasmon resonance interaction analysis. Specificity of FLT3L was measured using cell-based flow cytometry and an in vitro functional neutralization assay. ADCC activity was measured using an in vitro cell culture system. Toxicity and toxicokinetics were evaluated in cynomolgus monkeys during AMG 329 dosing (5-100 mg/kg; ≤ 27 weeks) and recovery (≤32 weeks). The AMG 329 antigen-binding region selectively bound to human and cynomolgus monkey FLT3L with affinities of 170 and 63 pM, respectively. AMG 329 specifically bound to and neutralized soluble and cell-bound human FLT3L and did not induce ADCC. AMG 329 administration generally reduced circulating plasmacytoid, conventional DC, and classical monocyte relative proportions in cynomolgus monkeys in a non-dose-dependent manner. Disruption of the FLT3/FLT3L signaling pathway presents a new potential therapeutic approach to treat autoimmune and inflammatory diseases. AMG 329 is a selective human monoclonal antibody antagonist of FLT3L that is currently being investigated in clinical studies.
Keywords: AMG 329; FLT3; FLT3 ligand; dendritic cells; monocytes; plasmacytoid dendritic cells.
Conflict of interest statement
Annie Lau-Kilby, Agata Bartczak, Susan Chyou, James Hester, Qian Wang, M. Jack Borrok and Kamelia Zerrouki are current full-time employees of Horizon Therapeutics (now Amgen) and may hold Horizon/Amgen stock or stock options. Michele Gunsior is a former full-time employee of Horizon (now Amgen) and AstraZeneca and may hold Horizon/Amgen and/or AstraZeneca stock or stock options, and is a current employee of Astria Therapeutics. Anna M. Hansen, Dorothy Sims, and Yan Chen are full-time employees of AstraZeneca, and may hold AstraZeneca stock or stock options. Xiaodong Xiao is a former employee of AstraZeneca and may hold AstraZeneca stock or stock options. Peter Pavlik is a former employee of AstraZeneca and a current employee of Aptevo Therapeutics. Kerry A. Casey is a former employee and shareholder of AstraZeneca and a current employee and shareholder of Regeneron Pharmaceuticals. William A. Rees is a former full-time employee of Horizon (now Amgen) and may hold Horizon/Amgen stock or stock options.
Figures

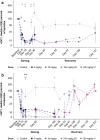
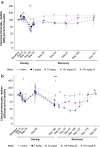
References
-
- Tobón GJ, Renaudineau Y, Hillion S, Cornec D, Devauchelle‐Pensec V, Youinou P, Pers J-O. The Fms-like tyrosine kinase 3 ligand, a mediator of B cell survival, is also a marker of lymphoma in primary Sjögren’s syndrome. Arthritis Rheumatism. 2010;62(11):3447–3456. doi: 10.1002/art.27611. - DOI - PubMed
-
- Saevarsdottir S, Olafsdottir TA, Ivarsdottir EV, Halldorsson GH, Gunnarsdottir K, Sigurdsson A, Johannesson A, Sigurdsson JK, Juliusdottir T, Lund SH, et al. FLT3 stop mutation increases FLT3 ligand level and risk of autoimmune thyroid disease. Nature. 2020;584(7822):619–623. doi: 10.1038/s41586-020-2436-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
