Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: Surgical Stability and Safety During a 2-Year Clinical Trial
- PMID: 40624842
- PMCID: PMC12235112
- DOI: 10.1111/ceo.14502
Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: Surgical Stability and Safety During a 2-Year Clinical Trial
Abstract
Background: To assess the safety and stability profile of the suprachoroidal retinal prosthesis (ScRP) in participants with retinitis pigmentosa (RP) for 2 years from implantation.
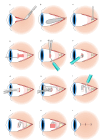
Methods: Four participants, with advanced RP and bare-light perception vision were enrolled in a prospective, single arm unmasked interventional clinical trial and unilaterally implanted with a 44-channel ScRP (NCT03406416). Electrical stimulation commenced in the psychophysics laboratory prior to use in local environments. Outcome measures included serious adverse events, adverse events, implant stability and implant functionality to assess the safety and stability profile over 2.0-2.7 years.
Results: Surgical procedures took 204-260 min and were uncomplicated. Postoperative recovery was uneventful. Imaging confirmed the device position under the macula and the absence of retinal trauma. There were no serious adverse events and the adverse events that occurred were mild. All electrodes were functional at surgery completion, and only 3% electrodes lost functionality by study end. There was minor array movement (translational and rotational) within the first 10-15 weeks only. The electrode to retina distance increased as expected with fibrous capsule development, but plateaued in three of four participants within 12 months. Retinal and choroidal thicknesses were consistent with the underlying retinal dystrophic disease.
Conclusions: The ScRP can be safely implanted in the suprachoroidal space and has minimal long-term impacts on the eye, with no SAEs and only slight array movement seen over 2.0-2.7 years. Hence, the findings indicate approach feasibility and further multicentre studies are warranted.
Keywords: adverse events; bionic eye; retinitis pigmentosa; suprachoroidal retinal prosthesis; surgical safety.
© 2025 The Author(s). Clinical & Experimental Ophthalmology published by John Wiley & Sons Australia, Ltd on behalf of Royal Australian and New Zealand College of Ophthalmologists.
Conflict of interest statement
P.J.A., D.A.X.N., J.V., C.E.W. and M.A.P. hold patents in relation to this work. P.J.A., M.K., E.K.B., S.A.T., J.K., D.A.X.N., C.D.L., M.A.P. and C.J.A. received financial support for the work from Bionic Vision Technologies. M.K., E.K.B., M.A.P. and C.J.A. received travel funding from Bionic Vision Technologies to present this work at an international conference in 2019. J.Y., R.J.B. and M.B.M. declare no conflicts of interest.
Figures





References
-
- Allen P. J., “Retinal Prostheses: Where to From Here?,” Clinical & Experimental Ophthalmology 49, no. 5 (2021): 418–429. - PubMed
-
- Luo Y. H.‐L. and Da Cruz L., “The Argus® II Retinal Prosthesis System,” Progress in Retinal and Eye Research 50 (2016): 89–107. - PubMed
-
- Hornig R., Dapper M., Le Joliff E., et al., “Pixium Vision: First Clinical Results and Innovative Developments,” in Artificial Vision: A Clinical Guide (Cham, Switzerland: Springer, 2017).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

