TROP2 Expression in Salivary Gland Adenoid Cystic Carcinoma (ACC) According to Histologic Subtype: Therapeutic Implications
- PMID: 40624990
- PMCID: PMC12419983
- DOI: 10.1111/jop.70008
TROP2 Expression in Salivary Gland Adenoid Cystic Carcinoma (ACC) According to Histologic Subtype: Therapeutic Implications
Abstract
Background: Adenoid cystic carcinoma (ACC) is a common salivary gland carcinoma with high recurrence and distant metastasis rates. Currently, there is no standard systemic treatment available. TROP2 is a transmembrane glycoprotein involved in the oncogenesis of several tumors that can be therapeutically targeted by a TROP2-antibody-drug conjugate (ADC). We aimed to characterize TROP2 expression in ACC and assess TROP2 as a potential therapeutic target.
Methods: TROP2 immunohistochemistry was performed in a tissue microarray including 165 ACC of salivary gland. The tumors were grouped according to the histological pattern as non-solid, solid + non-solid, or solid. TROP2 protein expression in ACC cell lines was assessed and subjected to drug screening with TROP2-ADC.
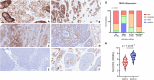
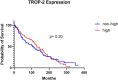
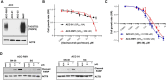
Results: TROP2 expression was high in 59%, moderate in 30%, weak in 8%, and negative in 3% of cases. TROP2 expression was significantly higher in non-solid compared with solid or solid + non-solid (p < 0.001). Notably, TROP2 expression was heterogenous among the dual cellular component, with TROP2 expression identified predominantly in the ductal and not in the myoepithelial cells. In vitro drug screening demonstrated that TROP2-ADC had selective anti-tumor effect in TROP2 expressing ACC cells.
Conclusions: TROP2 expression is prevalent in ACC, particularly in the ductal cell component of the non-solid tumors. The pre-clinical drug screening findings provide a biological rationale for exploring TROP2 as a therapeutic target in TROP2-expressing ACC.
Trial registration: clinicaltrials.gov: NCT05884320; NCI-2023-04260.
Keywords: ADC; SN‐38; TROP2; adenoid cystic carcinoma; antibody–drug conjugate; immunohistochemistry; sacituzumab govitecan.
© 2025 The Author(s). Journal of Oral Pathology & Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Ferrarotto reports personal fees from Regeneron, Sanofi, Merck Serono, Elevar Therapeutics, Prelude Therapeutics, Eisai Inc., Remix Therapeutics, and Coherus BioSciences; nonfinancial support from Ayala Pharmaceuticals, EMD Serono, ISA, Genentech/Roche, Merck Serono, Pfizer, Viracta, and Gilead outside the submitted work. Dr. Sousa reports being the recipient of funds given to MD Anderson Cancer Center by Elevar Therapeutics, Agenus, and Regeneron in the last 36 months. Dr. Matos reports fees from MSD (HPV‐vaccine) not related to the present study.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

