A comprehensive review on magnetic manganese as catalysts in organic synthesis
- PMID: 40625414
- PMCID: PMC12230672
- DOI: 10.1039/d5ra02493e
A comprehensive review on magnetic manganese as catalysts in organic synthesis
Abstract
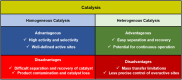
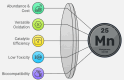
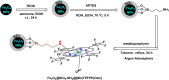
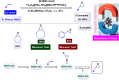
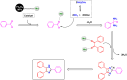
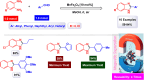
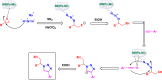
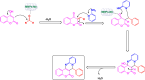
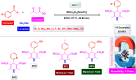
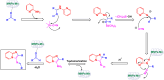
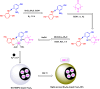
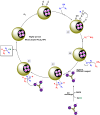
Manganese-based magnetic catalysts have gained significant attention in modern catalysis due to their unique combination of high catalytic efficiency, magnetic recoverability, and environmental sustainability. These catalysts, typically composed of manganese oxides, manganese-doped ferrites, or Mn-functionalized magnetic nanoparticles, facilitate a wide range of chemical transformations, including oxidation reactions, coupling reactions, and multicomponent reactions especially in the synthesis of heterocycles. Their ability to exhibit multiple oxidation states, strong redox activity, and high surface area makes them highly effective in selective and energy-efficient catalytic processes. Additionally, their magnetic properties enable easy separation from reaction mixtures using an external magnetic field, improving catalyst recyclability and reducing operational costs. Compared to conventional catalysts, magnetic manganese catalysts offer superior stability, cost-effectiveness, and eco-friendliness, making them promising alternatives for industrial-scale applications. This review explores recent advancements in the synthesis, mechanistic insights, and diverse applications of magnetic manganese catalysts, highlighting their role in sustainable and green chemistry. Furthermore, the challenges and future perspectives in optimizing their performance for broader catalytic applications are discussed. The insights presented in this review underscore the growing importance of magnetic manganese catalysts in developing efficient, cost-effective, and environmentally benign catalytic systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





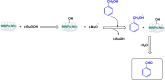
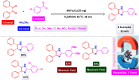
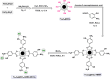
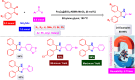









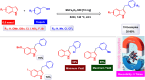
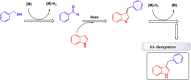
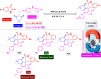

References
-
- Singh J. Juneja S. Soni R. K. Bhattacharya J. J. Colloid Interface Sci. 2021;590:60. - PubMed
-
- Moghadasi Z. Fajer N. Nanomater. Chem. 2024;1:130.
-
- Long Y. Zheng Y. Xia Y. Qu L. Yang Y. Xiang H. Zhou X. ACS Catal. 2022;12:4688.
-
- Singh J. Abraham R. Biol. Mol. Chem. 2024;2:13.
-
- Kim J. Hong S. H. ACS Catal. 2017;7:3336.
Publication types
LinkOut - more resources
Full Text Sources

