Silent Threats to the Liver: Acute Hepatotoxicity Attributed to Selective Androgen Receptor Modulators (SARMs)
- PMID: 40625511
- PMCID: PMC12230875
- DOI: 10.7759/cureus.85500
Silent Threats to the Liver: Acute Hepatotoxicity Attributed to Selective Androgen Receptor Modulators (SARMs)
Abstract
Selective androgen receptor modulators (SARMs) have garnered significant attention in recent years due to their potential to improve muscle mass and athletic performance with reduced androgenic side effects compared to traditional anabolic steroids. However, despite their growing popularity, there is increasing evidence linking SARMs to acute liver injury, raising concerns regarding their safety profile. SARMs can disrupt normal liver function through various pathways, including oxidative stress, alterations in lipid metabolism, and direct hepatocellular toxicity. This article explores the association between SARMs and acute liver injury, examining the mechanisms of hepatotoxicity, clinical manifestations, and potential therapeutic approaches. The findings underscore the need for further research and regulatory oversight in using SARMs, particularly in non-medical and performance-enhancing contexts.
Keywords: acute hepatotoxicity; acute liver injury; androgen receptors; drug-induced liver injury (dili); muscle growth supplements; non-medical use of sarms; painless jaundice; performance-enhancing drugs; sarms; sarms safety profile.
Copyright © 2025, Habeb et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
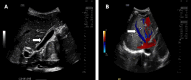
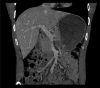
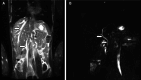
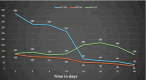
Figures
Similar articles
-
Selective Androgen Receptor Modulator-Induced Liver Injury in Active Duty Male.Mil Med. 2023 Jul 22;188(7-8):usac039. doi: 10.1093/milmed/usac039. Epub 2022 Mar 7. Mil Med. 2023. PMID: 35253885
-
Risk prediction of hepatotoxicity in paracetamol poisoning.Clin Toxicol (Phila). 2017 Sep;55(8):879-892. doi: 10.1080/15563650.2017.1317349. Epub 2017 Apr 27. Clin Toxicol (Phila). 2017. PMID: 28447858
-
Feasibility of implementing current best clinical practice for people who are using anabolic androgenic steroids within a Swiss primary care practice: a quality assurance study.Swiss Med Wkly. 2025 Feb 6;155:4225. doi: 10.57187/s.4225. Swiss Med Wkly. 2025. PMID: 39977451
-
Selective Androgen Receptor Modulators: An Emerging Liver Toxin.J Clin Transl Hepatol. 2023 Feb 28;11(1):188-196. doi: 10.14218/JCTH.2022.00207. Epub 2022 Nov 4. J Clin Transl Hepatol. 2023. PMID: 36479151 Free PMC article. Review.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
References
-
- Liver injury associated with the use of selective androgen receptor modulators and post-cycle therapy: two case reports and literature review. Koller T, Vrbova P, Meciarova I, Molcan P, Smitka M, Adamcova Selcanova S, Skladany L. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8180234/ World J Clin Cases. 2021;9:4062–4071. - PMC - PubMed
-
- S2851 harm by SARM: a case of drug-induced liver injury in an amateur bodybuilder. Akhtar N, Locke D, Stine J. https://journals.lww.com/ajg/Fulltext/2021/10001/S2851_Harm_by_SARM__A_C... Am J Gastroenterol. 2021;116:0.
-
- Selective androgen receptor modulators (SARMs)-induced liver injury: a case report and review of literature. Mohamed WT, Jahagirdar V, Fatima I, et al. https://pubmed.ncbi.nlm.nih.gov/36945289/ Cureus. 2023;15:0. - PMC - PubMed
-
- Selective androgen receptor modulators: an emerging liver toxin. Mohideen H, Hussain H, Dahiya DS, Wehbe H. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9647117/ J Clin Transl Hepatol. 2022;11:188–196. - PMC - PubMed
-
- RAD-140 drug-induced liver injury. Leung K, Yaramada P, Goyal P, Cai CX, Thung I, Hammami MB. https://www.ochsnerjournal.org/content/early/2022/07/13/toj.22.0005. Ochsner J. 2022;22:361–365. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
