A Novel Retinal Nerve Fiber Layer Biomarker of Amyotrophic Lateral Sclerosis (ALS) Identified Using Longitudinal in vivo Ocular Imaging
- PMID: 40625857
- PMCID: PMC12230754
- DOI: 10.2147/EB.S516163
A Novel Retinal Nerve Fiber Layer Biomarker of Amyotrophic Lateral Sclerosis (ALS) Identified Using Longitudinal in vivo Ocular Imaging
Abstract
Purpose: Like motor neurons, retinal ganglion cells (RGCs) have long axons and high metabolic demands, making them vulnerable to disruption of axonal transport. Unlike motor neurons, the RGC axons are accessible to high-resolution non-invasive optical imaging in their intraocular portion. A non-invasive in vivo retinal imaging biomarker can be valuable for amyotrophic lateral sclerosis (ALS) diagnosis and monitoring. We aim to assess the presence of inner retinal pathology in a mouse model of ALS and its possible progression with age.
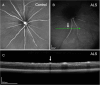
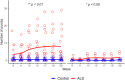
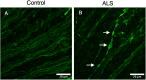
Methods: Transgenic SOD1G93A mice (n=8, 4M/4F) and age-matched controls (n=8, 4M/4F) underwent in vivo retinal imaging with confocal scanning laser ophthalmoscopy (cSLO) coupled with optical coherence tomography (OCT) at 20 weeks of age. Another group of SOD1G93A mice (n=20, 6M/14F) and age-matched controls (n=20, 6M/14F) underwent longitudinal in vivo retinal imaging with the same device. Each retinal imaging session included infrared reflectance (IR) and blue reflectance (BR) cSLO coupled with OCT. Hyperreflective puncta located in the retinal nerve fiber layer (RNFL) were counted in a blinded fashion in ALS and control mice. The number of puncta at 20 weeks of age in ALS mice was compared with controls using Wilcoxon test. The rates of increase of puncta number were analyzed using a Generalized Linear Mixed-Effect Model (GLMM) for genotype, time, and sex.
Results: IR-cSLO coupled with OCT revealed hyperreflective puncta located in the RNFL of ALS mice. IR-cSLO fundus imaging at the age of 20 weeks showed ALS mice had significantly higher number of puncta compared to controls (2.1±2.3 vs 0.5±0.8; (mean±SD), respectively, p=0.036). GLMM analysis showed both ALS mutation and age were significantly associated with the rate of increase of puncta number (p=0.000232 and p=0.000366, respectively). In addition, female ALS mice had a steeper increase of puncta compared to male ALS mice (0.21±0.04 log number puncta/week vs 0.16±0.04, respectively; p=0.037).
Conclusion: Our findings demonstrate distinct inner retinal nerve fiber layer pathology, detected using cSLO coupled with OCT, which worsens over time. These findings support the potential of retinal imaging as a translationally relevant, non-invasive biomarker for ALS diagnosis or disease monitoring in humans.
Keywords: amyotrophic lateral sclerosis; eye imaging; hyperreflective; mouse; optical coherence tomography; pathology; puncta; retinal nerve fiber layer; sex; spheroids; superoxide dismutase.
© 2025 Khorrami et al.
Conflict of interest statement
Dr Yeni Yucel reports lecture fees from Mitsubishi Tanabe Pharma Canada, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Ganglion Cell Layer Thickness as a Biomarker for Amyotrophic Lateral Sclerosis Functional Outcome: An OCT study.Rom J Ophthalmol. 2025 Apr-Jun;69(2):200-207. doi: 10.22336/rjo.2025.32. Rom J Ophthalmol. 2025. PMID: 40698100 Free PMC article.
-
Amyotrophic lateral sclerosis and retinal changes in optical coherence tomography: A systematic review and meta-analysis.Brain Behav. 2022 Sep;12(9):e2741. doi: 10.1002/brb3.2741. Epub 2022 Aug 22. Brain Behav. 2022. PMID: 35996223 Free PMC article.
-
Evaluating the impact of anti-CGRP monoclonal antibodies on retinal features in migraine patients: a retrospective optical coherence tomography study.Ther Adv Neurol Disord. 2025 Jun 24;18:17562864251347277. doi: 10.1177/17562864251347277. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 40568185 Free PMC article.
-
Regional retinal vulnerability in multiple sclerosis: integrating OCT, MRI, and clinical data for enhanced diagnosis and automated monitoring.Rom J Morphol Embryol. 2025 Jan-Mar;66(1):119-130. doi: 10.47162/RJME.66.1.11. Rom J Morphol Embryol. 2025. PMID: 40384198 Free PMC article.
-
Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis.Lancet Neurol. 2017 Oct;16(10):797-812. doi: 10.1016/S1474-4422(17)30278-8. Epub 2017 Sep 12. Lancet Neurol. 2017. PMID: 28920886
References
LinkOut - more resources
Full Text Sources
Miscellaneous

