High intake of n-6 polyunsaturated fatty acid exacerbates non-alcoholic steatohepatitis by the involvement of multiple metabolic pathways
- PMID: 40626231
- PMCID: PMC12229839
- DOI: 10.3389/fnut.2025.1562509
High intake of n-6 polyunsaturated fatty acid exacerbates non-alcoholic steatohepatitis by the involvement of multiple metabolic pathways
Abstract
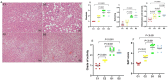
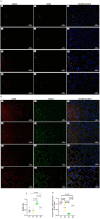
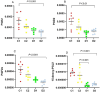
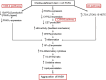
Non-alcoholic steatohepatitis (NASH) is characterized by steatosis, inflammation, and hepatocyte damage. A Western-style diet characterized by excessive n-6 polyunsaturated fatty acid (n-6 PUFA) intake, which is metabolized to pro-inflammatory arachidonic acids (AAs), might contribute to the exacerbation of NASH. Investigating the interactive effects of choline deficiency and n-6 PUFA supplementation on NASH progression, we aimed to elucidate how AA metabolites, such as leukotrienes, prostaglandins, and the CYP2J3/epoxyeicosatrienoic acids (EET) pathway influence disease pathogenesis. Rats were fed one of four diets: choline-sufficient with low n-6 PUFA and high saturated fatty acid (SFA) (C1), choline-sufficient with high n-6 PUFA (C2), choline-deficient with high n-6 PUFA (D1), or choline-deficient with low n-6 PUFA and high SFA (D2). Liver damage, inflammation, and oxidative stress in D1 were more than compared to C2 and D2 groups. Aggravation of NASH in D1 was accompanied by reduced levels of 15-deoxy-Δ12,14-prostaglandin J2 and PPAR-γ, weakening anti-inflammatory effects and lipid metabolism. Decreased CYP2J3 expression along with reduced PPAR-α levels, likely contributed to reduced anti-inflammatory EET levels, while elevated soluble epoxide hydrolase increased pro-inflammatory dihydroxyeicosatrienoic acids. Additionally, higher leukotriene C4 and 15-hydroxyeicosatetraenoic acid levels via the lipoxygenase pathway exacerbated inflammation. The combined pathway alterations in the D1 group increased inflammation, leading to elevated NF-κB expression, Kupffer cell polarization to M1, and lipid peroxidation, with n-6 PUFA interacting with choline deficiency to exacerbate these effects. Correlational analysis revealed significant associations between these pathways and inflammatory/oxidative markers. Our findings suggest that high intake of n-6 PUFA could aggravate NASH.
Keywords: arachidonic acid; choline deficiency; leukotrienes; lipoxygenase pathway; n-6 polyunsaturated fatty acids; non-alcoholic steatohepatitis.
Copyright © 2025 Jian-Tong, Zhou, Song, Yang, Fang, Liu, Cui, Lu, Zhu, Jin and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Warner JB, Zirnheld KH, Hu H, Floyd A, Kong M, McClain CJ, et al. Analysis of alcohol use, consumption of micronutrient and macronutrients, and liver health in the 2017-2018 National Health and nutrition examination survey. Alcohol Clin Exp Res. (2022) 46:2025–40. doi: 10.1111/acer.14944, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

