Information standards for innovative surgery: what patients need to know
- PMID: 40626615
- PMCID: PMC12235506
- DOI: 10.1093/bjs/znaf140
Information standards for innovative surgery: what patients need to know
Abstract
Background: There are repeated and ongoing failures in shared decision-making and informed consent for innovative surgical procedures. Governments and regulatory bodies internationally recommend establishing information standards to support safe and transparent surgical innovation. The aim of this study was to develop a core information set (CIS) for surgical innovation.
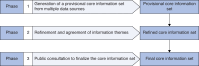
Methods: This was a mixed-method study in three phases: a provisional CIS was generated from multiple data sources (interviews with patients/professionals (44), recorded consultations (34), policy documents (58), and published studies (213)) using qualitative content analysis; the CIS was refined, with input from key stakeholders (patient representatives, surgeon innovators, anaesthetists, lawyers, ethicists, medical directors, academic experts, and regulatory representatives) using a modified nominal group technique; and the CIS was finalized through public consultation.
Results: The final CIS comprised seven themes that included: what is 'new' about the procedure; potential conflicts of interest; reasons for the innovation (including why the innovation is believed to be appropriate for the patient); treatment alternatives; unknowns (including uncertain safety/efficacy and that the procedure may be abandoned/modified); expertise with the innovation; and governance, oversight, and accountability (including how safety will be monitored and recompense if anything goes wrong). Two themes require follow-up discussions after the procedure.
Conclusion: A seven-theme CIS for surgical innovation was co-developed, with input from key stakeholders. International implementation of these information standards may support safe and transparent surgical innovation.
© The Author(s) 2025. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Conflict of interest statement
2.1 Statement of any relevant conflicts of interest.
Explanation: Conflicts of interest to be disclosed to provide transparency as to how personal or organizational interests relevant to the new procedure/device may influence decision-making.
Elaboration: Tailored discussions may include relevant financial, corporate, reputational, and emotional (for example enthusiasm) interests that may be relevant at an individual (for example surgeon) or organizational (for example department, hospital) level.
References
-
- Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 2022;7:991–1004 - PubMed
-
- Thompson JE, Egger S, Böhm M, Haynes AM, Matthews J, Rasiah K et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol 2014;65:521–531 - PubMed
-
- Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12:225–235 - PubMed
-
- Meakins JL. Innovation in surgery: the rules of evidence. Am J Surg 2002;183:399–405 - PubMed
-
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105–1112 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical