Consensus document on preoperative diagnostic procedures in breast lesions
- PMID: 40626666
- PMCID: PMC12236140
- DOI: 10.32074/1591-951X-1113
Consensus document on preoperative diagnostic procedures in breast lesions
Abstract
Currently, percutaneous sampling via core needle or vacuum-assisted biopsy is the primary choice to guide the management of patients with clinical or screen-detected breast lesions. Preoperative biopsies allow physicians to get pathological diagnoses as well as key prognostic and predictive data about the nature of the investigated process. Namely, adequate biopsy sampling is crucial for assigning lesions to one diagnostic category (B1-B5). Similarly, evaluating morphological (histotype, vascular invasion, necrosis, etc.) and immunohistochemical/molecular features (ER, PR, Ki-67, and HER2) is the key to address the most effective therapies, especially in the neoadjuvant setting. The multidisciplinary team should always discuss the results of percutaneous biopsies, whose global integration with clinical and radiological findings will drive the adoption of specific treatment options, particularly for uncertain (B3) and suspicious/malignant (B4-B5) lesions.
In the present work, we report a comprehensive overview of breast percutaneous biopsy techniques, diagnostic categories, and multidisciplinary management based on widely acknowledged evidence of good clinical practice.
Keywords: breast cancer; consensus; multidisciplinary management; preoperative biopsy.
Copyright © 2025 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The authors have nothing to disclose.
Figures


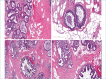
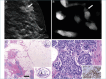
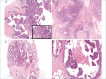
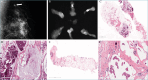



References
-
- Public Health England. Clinical guidance for breast cancer screening assessment (NHSBSP Publication No 49). London; 2016. Available at: https://www.gov.uk/government/publications/breast-screening-clinical-gui....
-
- Schünemann HJ, Lerda D, Quinn C, et al. Breast Cancer Screening and Diagnosis: A Synopsis of the European Breast Guidelines. Ann. Intern. Med. 2020;172:46-56. https://doi.org/10.7326/M19-2125. 10.7326/M19-2125 - DOI - PubMed
-
- Tamaki K, Sasano H, Ishida T, et al. Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci. 2010;101:2074-2079. https://doi.org/10.1111/j.1349-7006.2010.01630.x. 10.1111/j.1349-7006.2010.01630.x - DOI - PMC - PubMed
-
- Rageth CJ, O’Flynn EAM, Pinker K, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2019;174:279-296. https://doi.org/10.1007/s10549-018-05071-1. 10.1007/s10549-018-05071-1 - DOI - PMC - PubMed
-
- Sharma N, Cornford E, Cheung S, et al. The impact of vacuum-assisted excision in the management of indeterminate B3 lesions in the NHS Breast Screening Programme in England. Clin. Radiol. 2021;76:470.e23-470.e29. https://doi.org/10.1016/j.crad.2021.01.021. 10.1016/j.crad.2021.01.021 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

