Synthesis of 2-Azaanthraquinones from 1,4-Oxazinone Precursors
- PMID: 40626851
- PMCID: PMC12281559
- DOI: 10.1021/acs.joc.5c00881
Synthesis of 2-Azaanthraquinones from 1,4-Oxazinone Precursors
Abstract
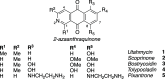
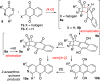
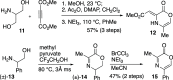
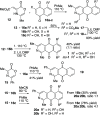
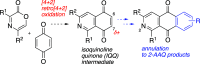
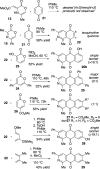
The synthesis of 2-azaanthraquinone structures from 1,4-oxazinone precursors is described. A new method for the synthesis of 1,4-oxazinones is also reported. The key discovery is the reaction of oxazinone and quinone starting materials through a tandem cycloaddition/cycloreversion sequence and in situ oxidation to deliver azaquinone products. Insights into the reactivity and selectivity of oxazinone precursors, as well as the description of new biologically active 2-azaanthraquinones further supplement this study.
Figures
References
-
- Abdelfattah M. S., Toume K., Arai M. A., Masu H., Ishibashi M.. Katorazone, a New Yellow Pigment with a 2-Azaquinone-Phenylhydrazone Structure Produced by Streptomyces Sp. IFM 11299. Tetrahedron Lett. 2012;53(26):3346–3348. doi: 10.1016/j.tetlet.2012.04.073. - DOI
-
- Arsenault G. P.. The Structure of Bostrycoidin, a β-Aza-Anthraquinone from Fusarium Solani D2 Purple. Tetrahedron Lett. 1965;6(45):4033–4037. doi: 10.1016/S0040-4039(01)99610-8. - DOI
-
- Gräfe U., Ihn W., Tresselt D., Miosga N., Kaden U., Schlegel B., Bormann E.-J., Sedmera P., Novák J.. Tolypocladin - a New Metal-Chelating 2-Aza-Anthraquinone from Tolypocladium Inflatum. Biol. Met. 1990;3(1):39–44. doi: 10.1007/BF01141176. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

