Metastable Macrocyclic Bis-Meisenheimer Adduct
- PMID: 40626993
- PMCID: PMC12435435
- DOI: 10.1002/anie.202511037
Metastable Macrocyclic Bis-Meisenheimer Adduct
Abstract
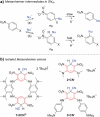
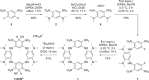
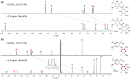
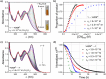
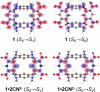
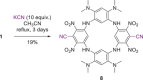
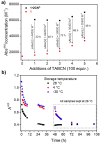
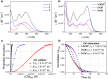
An electron-deficient tetranitroazacalixarene is shown to undergo reversible cyanide capture via nucleophilic aromatic substitution, yielding an unprecedented class of metastable dianionic macrocycle incorporating two Meisenheimer units, fully characterized by single-crystal X-ray diffraction, NMR, and electronic absorption spectroscopies. Acting as a chemical fuel, cyanide transiently drives the formation of this adduct, which can spontaneously regenerate the parent macrocycle under mild conditions, representing a rare demonstration of metastability in a Meisenheimer complex. The dynamic behavior of this system, reminiscent of out-of-equilibrium assemblies, is finely tunable through macrocycle concentration, counterion nature, solvent, and temperature. Detailed crystallographic, spectroscopic, and computational analyses reveal that intramolecular hydrogen bonding plays a key role in stabilizing the adduct. Comparative studies with simpler analogues further highlight the importance of macrocyclic preorganization and non-covalent interactions in governing this reversible reactivity.
Keywords: Chemical fuel; Dissipative system; Macrocycle; Meisenheimer complex; Nucleophilic aromatic substitution.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Meisenheimer J., Justus Liebigs Ann. Chem. 1902, 323, 205–246.
-
- Choi M. G., Kim N. Y., Lee Y. J., Ahn S., Chang S.‐K., Chem. Commun. 2019, 55, 11398–11401. - PubMed
-
- Rieth A. J., Gonzalez M. I., Kudisch B., Nava M., Nocera D. G., J. Am. Chem. Soc. 2021, 143, 14352–14359. - PubMed
-
- Calogero F., Wilczek L., Pinosa E., Gualandi A., Dorta R., Herrera A., Dai Y., Rossignol A., Negri F., Ziani Z., Fermi A., Ceroni P., Cozzi P. G., Angew. Chem. Int. Ed. 2024, 63, e202411074. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

