Optogenetic and chemical genetic tools for rapid repositioning of vimentin intermediate filaments
- PMID: 40627079
- PMCID: PMC12237251
- DOI: 10.1083/jcb.202504004
Optogenetic and chemical genetic tools for rapid repositioning of vimentin intermediate filaments
Abstract
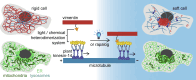
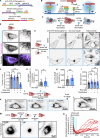
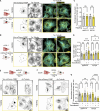
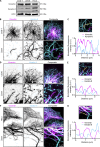
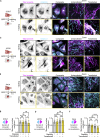
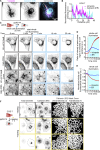
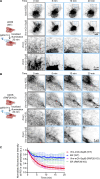
Intermediate filaments (IFs) are a key component of the cytoskeleton, essential for regulating cell mechanics, maintaining nuclear integrity, organelle positioning, and modulating cell signaling. Current insights into IF function primarily come from studies using long-term perturbations, such as protein depletion or mutation. Here, we present tools that allow rapid manipulation of vimentin IFs in the whole cytoplasm or within specific subcellular regions by inducibly coupling them to microtubule motors, either pharmacologically or using light. Rapid perinuclear clustering of vimentin had no major immediate effects on the actin or microtubule organization, cell spreading, or focal adhesion number, but it reduced cell stiffness. Mitochondria and endoplasmic reticulum (ER) sheets were reorganized due to vimentin clustering, whereas lysosomes were only briefly displaced and rapidly regained their normal distribution. Keratin moved along with vimentin in some cell lines but remained intact in others. Our tools help to study the immediate and local effects of vimentin perturbation and identify direct links of vimentin to other cellular structures.
© 2025 Pasolli et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








References
-
- Bargagna-Mohan, P., Hamza A., Kim Y.E., Khuan Abby Ho Y., Mor-Vaknin N., Wendschlag N., Liu J., Evans R.M., Markovitz D.M., Zhan C.-G., et al. 2007. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 14:623–634. 10.1016/j.chembiol.2007.04.010 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

