Safety, Tolerability, and Pharmacokinetics of NRL-1049, a Rho-Associated Kinase Inhibitor, in Healthy Volunteers: A Phase 1, First-in-Human, Single-Ascending Dose, Randomized, Placebo-Controlled Trial
- PMID: 40627117
- PMCID: PMC12354486
- DOI: 10.1007/s40263-025-01198-0
Safety, Tolerability, and Pharmacokinetics of NRL-1049, a Rho-Associated Kinase Inhibitor, in Healthy Volunteers: A Phase 1, First-in-Human, Single-Ascending Dose, Randomized, Placebo-Controlled Trial
Abstract
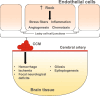
Background and objectives: Cerebral cavernous malformations (CCMs) are vascular lesions of the brain that can lead to hemorrhage, focal neurologic deficits, and seizures. Rho-associated kinase (ROCK) overactivation plays a critical role in the development of CCMs, and a novel, selective ROCK2 inhibitor, NRL-1049, mitigated lesion burden and bleeding in mouse models of CCM. This study examined the safety, tolerability, and pharmacokinetics of NRL-1049 in healthy volunteers.
Methods: In this first-in-human, randomized, double-blind, single-ascending dose study, participants received a single, oral dose of NRL-1049 (25, 75, 150, or 250 mg) or placebo in a fasted state (period 1). In period 2, participants received 150 mg NRL-1049 or placebo 30 min after a standardized high-fat, high-calorie meal. Blood samples for pharmacokinetic analysis were collected pre-dose and at post-dose time points from 5 min to 48 h. Treatment-emergent adverse events (TEAEs) were recorded and pharmacokinetic parameters determined, including maximum drug concentration (Cmax), time to Cmax (tmax), and area under the concentration-time curve (AUC) from time 0 to last quantifiable concentration (AUC0-t) and extrapolated to infinity (AUC0-∞).
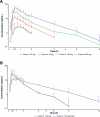
Results: Of the 24 participants in period 1 who received NRL-1049 (fasted), 9 (37.5%) experienced ≥ 1 TEAE, with 8 (33.3%) reporting ≥ 1 treatment-related TEAE. TEAEs appeared to correlate with dose, and 150 mg was the maximum tolerated dose following single-dose administration in this study. The most common TEAEs (> 5%) were dizziness (16.7%), headache (8.3%), and syncope (8.3%). In period 2 (n = 10), four (40.0%) participants who received 150 mg NRL-1049 (fed) reported ≥ 1 TEAE, and three (30.0%) reported a treatment-related TEAE. There were no reports of serious TEAEs or discontinuations due to a TEAE. NRL-1049 was rapidly absorbed in the fasted state, with median tmax ranging from 0.50 to 0.75 h. Mean Cmax increased over the dose range of 25-250 mg (3.66-58.0 ng/mL). As NRL-1049 dose increased in a ratio of 1:3:6:10, mean Cmax similarly increased (1:5:10:16), while AUC0-t and AUC0-∞ increased in a greater-than-dose proportional manner (1:5:11:25 and 1:4:10:21, respectively; P < 0.001). In the fed state (150 mg NRL-1049), mean Cmax (18.5 ng/mL) was lower compared with the fasted state (34.9 ng/mL). For the active metabolite, NRL-2017, in the fasted state, median tmax was 0.88-1.63 h, and mean Cmax increased over the dose range (54.2-1520 ng/mL). Mean Cmax (1:6:14:28), AUC0-t (1:4:7:14), and AUC0-∞ (1:3:6:13) of NRL-2017 increased in a greater-than-dose proportional manner (P < 0.001). In the fed state, mean Cmax was lower compared with the fasted state.
Conclusions: The maximum tolerated dose of 150 mg NRL-1049 was associated with a favorable safety profile in healthy adult volunteers. Exposure of NRL-1049 and its active metabolite, NRL-2017, increased in a dose proportional or greater-than-dose proportional manner. These results support continued investigation and development of NRL-1049.
Plain language summary
Cerebral cavernous malformations, or CCMs, are a type of abnormal formation of small blood vessels in the brain. Some CCMs may bleed and might lead to disability and seizure. NRL-1049 is a drug that showed promise treating CCMs in animal models. This first-in-human study looked at the safety of a single, oral dose of NRL-1049 in healthy volunteers. The body converts some NRL-1049 into NRL-2017, which works in a similar way. This study also looked at how long both drugs remain in the body. In the first part of this study, healthy volunteers took one of four different doses of NRL-1049, from 25 to 250 mg, or placebo on an empty stomach. Another group received 150 mg NRL-1049 or placebo 30 min after eating a high-fat meal. Most side effects with NRL-1049, such as dizziness or headache, were mild to moderate, and all resolved by the end of the study. NRL-1049 was quickly absorbed after dosing. Levels of NRL-1049 in the blood peaked within 1 h after dosing. NRL-1049 could be measured in the blood for up to 8 h on an empty stomach and 12 h after eating. NRL-2017 could be measured up to 48 h after taking NRL-1049. In all, a single, oral dose of NRL-1049 was generally safe and well tolerated in healthy volunteers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study was funded by Neurelis, Inc. (San Diego, California). Open access was funded by Neurelis, Inc. (San Diego, California). Conflicts of Interest: S.M. is an employee of and has received stock and stock options from Neurelis, Inc. I.A. is a consultant to Neurelis, Inc.; is chairman (unpaid) of the scientific advisory board for the Alliance to Cure Cavernous Malformation; and receives research support from the National Institute of Neurological Disorders and Stroke. M.A.L.T. is an employee of and has received stock options from Neurelis, Inc. L.M. is a consultant to Neurelis, Inc. J.G. was, at the time of this study, an employee of and has received stock options from Neurelis, Inc. L.Y.N. is an employee of and has received stock options from Neurelis, Inc. E.C. is an employee of and has received stock and stock options from Neurelis, Inc. A.L.R. is an employee of and has received stock options from Neurelis, Inc. Availability of Data and Material: All data are included in the manuscript. Ethics Approval: The study protocol, informed consent, and all other study documents were approved by an institutional review board (Advarra; protocol number, Pro00068446). The study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation, Guideline E6 for Good Clinical Practice (GCP), the Food and Drug Administration GCP Code of Federal Regulations Title 21 (part 56), the European regulation EU 536/2014, and the Tri-Council Policy Statement (Canada). Consent to Participate: Written consent was obtained from each participant prior to study enrollment. Consent for Publication: Not applicable. Code Availability: Not applicable. Author Contributions: S.M., M.A.L.T., J.G., E.C., and A.L.R. were involved in the conception and design of the study, as well as data analysis. All authors were involved in data interpretation, review of literature, and drafting of the manuscript. All authors critically reviewed and revised the work, and all read and approved the final version.
Figures


References
-
- Morrison L, Gutierrez J, Ayata C, et al. Current and future treatment options for cerebral cavernous malformations. Stroke Vasc Interv Neurol. 2024;4:1–11.
-
- Santos AN, Rauschenbach L, Saban D, et al. Multiple cerebral cavernous malformations: clinical course of confirmed, assumed and non-familial disease. Eur J Neurol. 2022;29:1427–34. - PubMed
-
- Santos AN, Rauschenbach L, Saban D, et al. Natural course of cerebral cavernous malformations in children: a five-year follow-up study. Stroke. 2022;53:817–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

