Ameliorative role of silver nanoparticles incorporated with chitosan solution and leukocyte platelet-rich fibrin scaffold during colon anastomosis in rabbits
- PMID: 40627202
- PMCID: PMC12238072
- DOI: 10.1007/s10856-025-06908-0
Ameliorative role of silver nanoparticles incorporated with chitosan solution and leukocyte platelet-rich fibrin scaffold during colon anastomosis in rabbits
Abstract
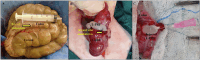
This research aimed to detect the experimental value of silver nanoparticles (AgNPs) loaded with chitosan (CS) and leukocyte platelet-rich fibrin (L-PRF) in promoting the healing process of the anastomotic colon in rabbits. Forty-two healthy male white New Zealand rabbits underwent total transection and anastomosis of the ascending colon. The rabbits were then randomly divided and equally allocated into two groups (n = 21): the control (C) group, in which the transected colon was traditionally anastomosed with simple interrupted sutures, and the treated (T) group, in which the colon was traditionally anastomosed and completely wrapped by a L-PRF scaffold that was inoculated with 1 ml of AgNPs-CS solution. Clinical macroscopic observations of rabbits and pain scale scores were evaluated postoperatively on the 7th,14th, and 28th days. Additionally, seven rabbits from each group were euthanized at each time point to assess bursting pressure, radiographic stenotic degree, histopathological findings, and immunohistochemical examination. The anastomotic colon in the T-group revealed a significant improvement in the overall health status of rabbits, as well as mechanical bursting pressure scores. Pain scores and radiographic stenotic degree were significantly lower in the T-group. Moreover, the histopathological examination of the anastomotic colon in the T-group showed an increased collagen deposition in the submucosa, proper re-epithelialization of the mucosa, higher angiogenesis, reduced adhesions, and significantly higher histopathological healing scores than the C-group. The AgNPs-CS/L-PRF scaffold successfully reduced complications following colon anastomosis and potentially ameliorated the healing process of the anastomotic colon in the experimental rabbits.
© 2025. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: The authors declare no competing interests. Ethics approval: All experiments were performed following relevant guidelines and regulations. This study was approved by the Ethics Committee of Mansoura University Animal Care and Use Committee with the code number MU-ACUC (VM.PhD.22.11.6). All procedures in this study were performed following ARRIVE guidelines and carried out in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and associated guidelines.
Figures












References
-
- Choi H-K, Law W-L, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006;49:1719–25. - PubMed
-
- Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am. 1997;77:549–73. - PubMed
-
- Hoeppner J, Willa K, Timme S, Tittelbach-Helmrich D, Hopt U, Keck T, et al. Reinforcement of colonic anastomoses with a collagenous double-layer matrix extracted from porcine dermis. Eur Surg Res. 2010;45:68–76. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

