Protection Against Persistent HPV-16/18 Infection After Different Number of Doses of Quadrivalent Vaccine in Girls and Young Women: A Randomized Clinical Trial
- PMID: 40627355
- PMCID: PMC12238905
- DOI: 10.1001/jamanetworkopen.2025.19095
Protection Against Persistent HPV-16/18 Infection After Different Number of Doses of Quadrivalent Vaccine in Girls and Young Women: A Randomized Clinical Trial
Abstract
Importance: There are no randomized studies comparing the long-term effectiveness of a 2-dose schedule (administered at 0 and 6 months) of quadrivalent human papillomavirus vaccine (4vHPV) with a 2 + 1-dose schedule (administered at 0, 6, and 60 months) given to preadolescents for the prevention of persistent HPV-16 and HPV-18 infection.
Objective: To evaluate whether a 2-dose 4vHPV schedule is noninferior to a 2 + 1-dose schedule for preventing persistent HPV-16 and HPV-18 infection up to 13 years after the first dose.
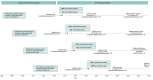
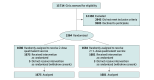
Design, setting, and participants: This 2-arm, parallel, noninferiority, randomized clinical trial (RCT), called ICI-VPH (Impact of HPV Immunization Schedules Against HPV), was conducted in Québec, Canada, from 2013 to 2021. Eligible participants were girls who had received 2 doses of 4vHPV, 6 months apart when they were aged 9 to 11 years, 5 years prior to trial recruitment. Efficacy-evaluable population analysis was conducted between April 2023 and June 2024.
Interventions: Participants were randomized 1:1 to the 2-dose or 2 + 1-dose groups. The 2 + 1-dose group received a booster dose of 4vHPV at the recruitment visit. Both groups were instructed on how to self-collect a vaginal sample, provided an initial vaginal swab, and completed an online questionnaire.
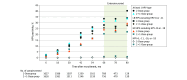
Main outcomes and measures: The primary outcome was persistent HPV-16 and HPV-18 infection, defined as the presence of HPV-16 or HPV-18 DNA in 2 consecutive vaginal samples self-collected 5 to 15 months apart.
Results: Among the 3356 participants, the mean (SD) age at enrollment was 14.8 (0.4) years, and 2867 (85.4%) were French Canadian individuals. Of the 3364 participants initially randomized, 8 (0.2%) withdrew immediately after randomization, 1675 (49.8%) were assigned to the 2-dose group, and 1681 (50.0%) were assigned to the 2 + 1-dose group. Of the 16 989 genotyping tests analyzed, 31 (0.2%) detected vaccine-type HPV-6, -11, -16, and -18. Only 1 participant in the 2 + 1-dose group (0.1%) had a time-limited persistent HPV-16 infection.
Conclusions and relevance: This clinical trial found that the incidence of HPV-16 and HPV-18 infection was low with both the 2-dose and 2 + 1-dose 4vHPV schedules. A 2-dose schedule provided robust protection against persistent HPV-16 and HPV-18 infection for up to 13 years, suggesting that a booster dose administered at 60 months would not provide additional benefit.
Trial registration: ClinicalTrials.gov Identifier: NCT02009800.
Conflict of interest statement
Figures
References
-
- World Health Organization . Human papillomavirus vaccines: WHO position paper, December 2022. Wkly Epidemiol Rec. 2022;97(50):645-672. https://www.who.int/publications/i/item/who-wer9750-645-672
-
- World Health Organization . Safety of HPV vaccines. July 14, 2017. Accessed October 27, 2024. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/t...
-
- Merck Canada Inc . GARDASIL—quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine. March 10, 2015. Accessed March 11, 2024. https://pdf.hres.ca/dpd_pm/00029689.PDF
-
- GlaxoSmithKline Inc . CERVARIX—human papillomavirus vaccine types 16 and 18 (recombinant, AS04 adjuvanted). February 3, 2010. Accessed March 11, 2024. https://ca.gsk.com/media/6236/cervarix.pdf
-
- Comité sur l’immunisation du Québec, Comité scientifique ad hoc VPH. La vaccination contre les VPH au Québec: mise à jour des connaissances et propositions du comité d’experts. Institut National de Santé Publique du Québec. 2012:148. Accessed January 23, 2025. https://www.inspq.qc.ca/publications/1518
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

