Oxidative stress is a shared characteristic of ME/CFS and Long COVID
- PMID: 40627396
- PMCID: PMC12280928
- DOI: 10.1073/pnas.2426564122
Oxidative stress is a shared characteristic of ME/CFS and Long COVID
Abstract
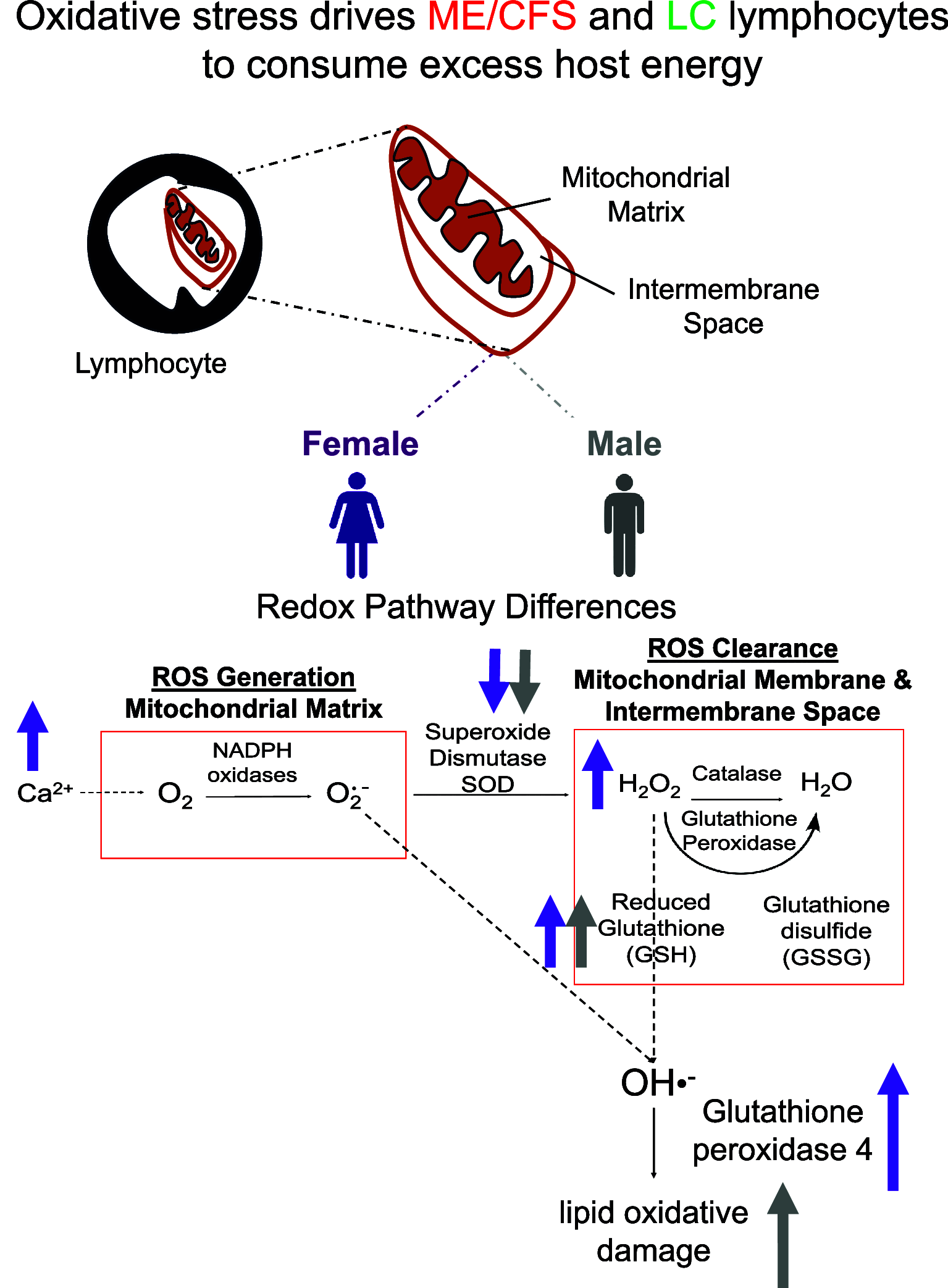
Over 65 million individuals worldwide are estimated to have Long COVID (LC), a complex multisystemic condition marked by fatigue, post-exertional malaise, and other symptoms resembling myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). With no clinically approved treatments or reliable diagnostic markers, there is an urgent need to define the molecular underpinnings of these conditions. By studying bioenergetic characteristics of peripheral blood lymphocytes in 25 healthy controls, 27 ME/CFS, and 20 LC donors, we find both ME/CFS and LC donors exhibit signs of elevated oxidative stress, especially in the memory subset. Using a combination of flow cytometry, RNA-seq, mass spectrometry, and systems chemistry analysis, we observed aberrations in reactive oxygen species (ROS) clearance pathways including elevated glutathione levels, decreases in mitochondrial superoxide dismutase protein levels, and glutathione peroxidase 4-mediated lipid oxidative damage. Strikingly, these redox pathways changes show sex-specific trends. While ME/CFS females exhibit higher total ROS and mitochondrial calcium levels, males have normal ROS levels, with pronounced mitochondrial lipid oxidative damage. In females, these higher ROS levels correlate with T cell hyperproliferation, consistent with the known role of elevated ROS in initiating proliferation. This hyperproliferation can be attenuated by metformin, suggesting this Food and Drug Administration (FDA)-approved drug as a possible treatment, as also suggested by a recent clinical study of LC patients. Moreover, these results suggest a shared mechanistic basis for the systemic phenotypes of ME/CFS and LC, which can be detected by quantitative blood cell measurements, and that effective, patient-tailored drugs might be discovered using standard lymphocyte stimulation assays.
Keywords: ME/CFS; fatigue; long COVID; metabolism; oxidative stress.
Conflict of interest statement
Competing interests statement:S.S. co-founded and is a scientist at Material Alchemy (MA), an independent entity for Designing Materials for Sustainability. Turium was developed by MA for analyzing complex systems chemistry and is available to academia for research with licensing. V.S., M.S., S.S., P.S.M., H.B., and M.M.D. are inventors on a patent related to oxidative stress signatures in ME/CFS and LC. M.S. is a cofounder and scientific advisor of Crosshair Therapeutics, Exposomics, Filtricine, Fodsel, iollo, InVu Health, January AI, Marble Therapeutics, Mirvie, Next Thought AI, Orange Street Ventures, Personalis, Protos Biologics, Qbio, RTHM, SensOmics. M.S. is a scientific advisor of Abbratech, Applied Cognition, Enovone, Jupiter Therapeutics, M3 Helium, Mitrix, Neuvivo, Onza, Sigil Biosciences, TranscribeGlass, WndrHLTH, Yuvan Research. M.S. is a cofounder of NiMo Therapeutics. M.S. is an investor and scientific advisor of R42 and Swaza. M.S. is an investor in Repair Biotechnologies.
Figures




Update of
-
Oxidative Stress is a shared characteristic of ME/CFS and Long COVID.bioRxiv [Preprint]. 2024 May 5:2024.05.04.592477. doi: 10.1101/2024.05.04.592477. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2426564122. doi: 10.1073/pnas.2426564122. PMID: 38746454 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

