Hem1 controls T cell activation, memory, and the regulated release of immunosuppressive and proinflammatory cytokines
- PMID: 40627451
- PMCID: PMC12406723
- DOI: 10.1172/jci.insight.174235
Hem1 controls T cell activation, memory, and the regulated release of immunosuppressive and proinflammatory cytokines
Abstract
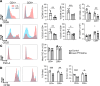
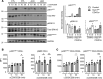
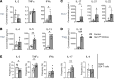
Hematopoietic protein-1 (Hem1) is a component of the WASp family verprolin-homologous protein (WAVE) actin regulatory complex, which is activated downstream of multiple immune receptors. Mutations in the NCKAP1L gene encoding HEM1 have recently been found to result in severe primary immunodeficiency disease (PID), characterized by recurrent respiratory infections, hyperinflammation, autoimmunity, and high mortality. However, how loss of Hem1 results in PID is unclear. To define the importance of Hem1 specifically in T cells, we generated constitutive and T cell-specific Hem1-null mice. Hem1-deficient T cells exhibited an increased shift from naive to memory T cells and increased ratio of immunosuppressive regulatory to effector T cells. Loss of Hem1 resulted in hallmarks of T cell exhaustion, including T cell lymphopenia, decreased activation and proliferation, increased expression of PD-1 and Tim3, and increased IL-10 production. In vitro TCR stimulation of CD4+ T cells resulted in increased production of Th1 (IFN-γ), Th2 (IL-5, IL-13), Th17 (IL-17, IL-22), and Treg (IL-10) cytokines. This correlated with reduced F-actin, increased expression of CD107a, and increased granzyme release indicative of increased granule membrane fusion and exocytosis. These results suggest that Hem1 is critical for maintaining T cell activation, homeostasis, and regulated cytokine production following antigen encounter.
Keywords: Adaptive immunity; Cytoskeleton; Immunology; Inflammation; T cells.
Conflict of interest statement
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

