Phase Ib Trial of Fulvestrant, Palbociclib, and Erdafitinib, a pan-FGFR Tyrosine Kinase Inhibitor, in HR+/HER2- Metastatic Breast Cancer
- PMID: 40627530
- PMCID: PMC12326521
- DOI: 10.1158/1078-0432.CCR-24-3803
Phase Ib Trial of Fulvestrant, Palbociclib, and Erdafitinib, a pan-FGFR Tyrosine Kinase Inhibitor, in HR+/HER2- Metastatic Breast Cancer
Abstract
Purpose: We report herein a phase Ib trial to determine the safety, tolerability, and antitumor activity of erdafitinib, a pan-FGFR tyrosine kinase inhibitor, with fulvestrant and palbociclib in patients with hormone receptor-positive/HER2-negative metastatic breast cancers (NCT03238196).
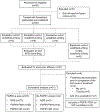
Patients and methods: Thirteen patients were enrolled on the escalation phase in a traditional 3 + 3 trial design to determine the maximum tolerated dose (MTD). Subsequently, 22 patients were treated at the established MTD during the expansion phase. All patients had received prior treatment with cyclin-dependent kinase-4/6 inhibitors and endocrine therapy, and 29 showed FGFR pathway alterations in their tumors.
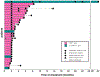
Results: The MTD of erdafitinib was 6 mg taken orally once daily when combined with palbociclib and fulvestrant. The triple combination showed clinically manageable tolerability. Most common adverse events were neutropenia, likely attributable to palbociclib, and oral mucositis and hyperphosphatemia, attributable to erdafitinib. Three patients showed a partial response, one of them lasting more than 2.5 years, despite lacking detectable FGFR1 to FGFR4 somatic alterations. FGFR1 amplification was not associated with response to FGFR inhibition, but high FGFR1 protein expression, measured by IHC, correlated with longer progression-free survival within the FGFR1-amplified cohort. There was no correlation between FGFR1 copy number and FGFR1 protein levels in specimens from metastatic sites, potentially highlighting the need for a more recent metastatic tumor biopsy for biomarker evaluation.
Conclusions: The trial endpoint was met establishing the MTD of erdafitinib at 6 mg. Whereas the triplet regimen may pose tolerability challenges, alterative doublets with selective FGFR1 inhibitors in patients with FGFR1-dependent tumors, possibly administered in sequence, are worthy of further investigation.
©2025 American Association for Cancer Research.
Conflict of interest statement
JMB receives research support from Genentech/Roche and Incyte Corporation, has received advisory board payments from AstraZeneca, Eli Lilly, and Mallinckrodt and is an inventor on patents regarding immunotherapy targets and biomarkers in cancer. BHP is a paid scientific advisory board member for Celcuity Inc., and an unpaid consultant for Tempus Inc, and has research contracts with Tempus, Caris and Guardant Health. Under separate licensing agreements between Horizon Discovery, LTD and The Johns Hopkins University, B.H.P. is entitled to a share of royalties received by the University on sales of products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict-of-interest policies. IAM is an AstraZeneca employee. CLA has received research funding from Pfizer and Lilly. The other authors declare no potential conflicts of interest.
Figures

References
-
- Loibl S, et al. , Breast cancer. Lancet, 2021. 397(10286): p. 1750–1769. - PubMed
-
- Dowsett M, Nicholson RI, and Pietras RJ, Biological characteristics of the pure antiestrogen fulvestrant: overcoming endocrine resistance. Breast cancer research and treatment, 2005. 93: p. 11–18. - PubMed
-
- Dean J, et al. , Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene, 2010. 29(28): p. 4018–4032. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

