Fetal hemoglobin enables malaria parasite growth in sickle cells but augments production of transmission stage parasites
- PMID: 40627625
- PMCID: PMC12237050
- DOI: 10.1371/journal.pone.0325797
Fetal hemoglobin enables malaria parasite growth in sickle cells but augments production of transmission stage parasites
Abstract
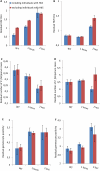
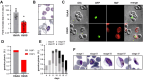
Sickle cell trait is the quintessential example of the human evolutionary response to malaria, providing protection against severe disease, but leading to sickle cell disease (SCD) in the homozygous state. Fetal Hemoglobin (HbF) reduces the pathology of SCD and several mutations lead to the prolonged production of HbF into childhood and adult life. HbF has been suggested to contribute to protection against malaria. Two long-term cohorts were genotyped for three quantitative trait loci associated with HbF production and analyzed for HbF titers, malaria clinical episodes and the production of parasite stages infectious to mosquitoes, gametocytes, in asymptomatic infections. Plasmodium falciparum parasites were also grown in vitro in HbSS cells with measured levels of HbF. The genetic determinants of prolonged HbF production were associated with increased HbF titers and that increased HbF afforded protection from malaria disease but increased the production of gametocytes. The presence of HbF in sickled red cells was also shown in in vitro culture to enable parasite persistence in conditions otherwise deleterious for the parasite and enabled complete maturation of gametocytes. The beneficial personal effect of HbF, whether through protection against malaria or alleviating effects of SCD, is seemingly offset by increased parasite transmissibility and potential disease burden for the community. These individuals represent a potentially important reservoir of infection and could be targeted in elimination strategies.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

