WERF Endometriosis Phenome and Biobanking Harmonisation Project for Experimental Models in Endometriosis Research (EPHect-EM-Organoids): endometrial organoids as an emerging technology for endometriosis research
- PMID: 40628401
- PMCID: PMC12237518
- DOI: 10.1093/molehr/gaaf024
WERF Endometriosis Phenome and Biobanking Harmonisation Project for Experimental Models in Endometriosis Research (EPHect-EM-Organoids): endometrial organoids as an emerging technology for endometriosis research
Abstract
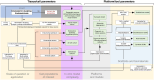
The aetiology of endometriosis remains poorly understood. In vitro model systems provide the opportunity to identify the mechanisms driving disease pathogenesis using human cells. Three-dimensional models, particularly organoid systems, have revolutionized how we study epithelial biology and are powerful tools for modelling endometriosis. As an emerging model system, it is important to define protocols and identify the remaining challenges surrounding endometrial organoid culture to increase reproducibility and scientific rigour in endometriosis research. The World Endometriosis Research Foundation (WERF) established an international working group comprised of experts using in vitro approaches for the study of endometriosis. This working group harmonized protocols and documentation of existing and emerging organoid systems to maximize comparison and replication across the field and guide specific research hypotheses testing. This evaluation of organoid protocols, limitations, challenges, and alternative approaches assessed both published and grey literature papers across several disciplines pertinent to endometriosis research. Recommendations for protocol and documentation harmonization are presented, and we created the first-ever decision tree diagram to guide and facilitate the selection of existing models best suited for specific areas of endometriosis research. Rigorous and systematic assessment of emerging organoid systems, recognizing the inferential strengths and limitations of these approaches, is vital for endometriosis research. This comprehensive review of the benefits, limitations, and utilization of organoid models, as well as the consequent integration of protocols and documentation, will contribute to the scientific knowledge base by maximizing the reproducibility, comparability, and interpretation of research studies in endometriosis. Additionally, these newly developed protocols and documentation should serve as a resource for, and facilitate collaboration between, endometriosis investigators using organoids in their research methods.
Keywords: collaboration; emerging models; endometriosis; experimental models; organoids; research.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
S.A.M. is a trustee of WERF, co-principal investigator of EPHect, a past president of the World Endometriosis Society (WES), and a member of the scientific advisory board of NextGen Jane; she has received presentation remuneration from Gideon Richter, and research funding from AbbVie, LLC, the Marriott Family Foundations, the USA National Institutes of Health (NIH), and the USA Peer Reviewed Medical Research Program (PRMRP), none of which is related to this work. K.L.B.-T. is a trustee of WERF. E.G. is a member of the scientific advisory board of FimmCyte AG and a trustee of WERF. L.H. receives remuneration from WERF as the EPHect programme manager and has provided consultancy to Gesynta Pharma AS, which has no bearing on this work. J.S.G., E.E.M., S.M.H., and K.G.O. declare no conflicts of interest.
Figures



References
-
- Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res 2018;52:751–762. - PubMed
-
- Arnold JT, Kaufman DG, Seppälä M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 2001;16:836–845. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

