The PARP inhibitor talazoparib synergizes with reovirus to induce cancer killing and tumour control in vivo in mouse models
- PMID: 40628717
- PMCID: PMC12238577
- DOI: 10.1038/s41467-025-61297-w
The PARP inhibitor talazoparib synergizes with reovirus to induce cancer killing and tumour control in vivo in mouse models
Abstract
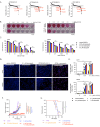
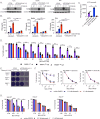
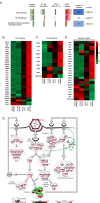
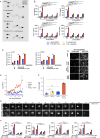
Reovirus type 3 Dearing (RT3D) is an oncolytic, double-stranded RNA virus. To identify potential RT3D drug-viral sensitizer, here we use a high-throughput screen of therapeutic agents and find a PARP-1 inhibitor, talazoparib, as a top hit. RT3D interacts with retinoic acid-induced gene-1 (RIG-I) and activates PARP-1, with consequent PARylation of components of the extrinsic apoptosis pathway. Pharmacological or genetic inhibition of PARP-1 abrogates this PARylation and enhances extrinsic apoptosis, NF-kB signalling and pro-inflammatory cell death. Interaction between PARP-1 and RIG-I induced by treating RT3D-infected cells with talazoparib activates downstream IFN-β and TNF/TRAIL production to amplify the therapeutic effect through positive feedback. Furthermore, the effect of RT3D-talazoparib combination is phenocopied by non-viral ds-RNA therapy and RIG-I agonism. In vivo, mouse tumour model results show that RT3D/talazoparib combination regimen induces complete control of inoculated tumour as well as protection from subsequent tumour rechallenge with the, with accompanied innate and adaptive immune activation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Kim, S. G. et al. Phase II trial of pexa-vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia virus, in patients with metastatic, refractory renal cell carcinoma (RCC). J. Clin. Oncol.36 (2018). Meeting Abstract: 2013 ASCO Annual Meeting.
-
- Cui, C. L. et al. OrienX010 oncolytic viral therapy in phase Ic trial of intralesional injection in liver metastases among patients with stage IV melanoma after standard treatment. J. Clin. Oncol.35, e21013 (2017).
-
- Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol.33, 2780–2788 10.1200/JCO.2017.35.15_suppl.e21013 (2015). - PubMed
-
- Pecora, A. L. et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol.20, 2251–2266 (2002). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

