Structural insights into GPCR signaling activated by peptide ligands: from molecular mechanism to therapeutic application
- PMID: 40629042
- PMCID: PMC12322139
- DOI: 10.1038/s12276-025-01497-y
Structural insights into GPCR signaling activated by peptide ligands: from molecular mechanism to therapeutic application
Abstract
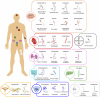
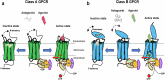
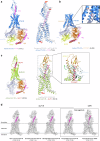
Recent advances in structural biology have profoundly enhanced our understanding of G protein-coupled receptors (GPCRs), providing detailed molecular insights into their activation and ligand recognition. Here, in this Review, we explore the molecular mechanisms of class A and class B GPCRs bound to peptide agonists and their implications for drug development. We examine representative GPCRs, such as the angiotensin II type 1 receptor, chemokine receptor 5, μ-opioid receptor, parathyroid hormone 1 receptor and glucagon-like peptide 1 receptor (GLP-1R), highlighting their activation processes upon peptide ligand binding. Comparative analysis of structures bound to endogenous and synthetic peptide ligands reveals critical insights for rational drug design. A case study on GLP-1R demonstrates how structural insights have led to the design of successful drugs for type 2 diabetes and obesity. This comparative structural analysis aims to deepen our understanding of GPCR activation mechanisms and support future drug discovery efforts targeting peptide-binding GPCRs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Computational modelling of dynamic cAMP responses to GPCR agonists for exploration of GLP-1R ligand effects in pancreatic β-cells and neurons.Cell Signal. 2024 Jul;119:111153. doi: 10.1016/j.cellsig.2024.111153. Epub 2024 Mar 30. Cell Signal. 2024. PMID: 38556030
-
Docking Simulations of G-Protein Coupled Receptors Uncover Crossover Binding Patterns of Diverse Ligands to Angiotensin, Alpha-Adrenergic and Opioid Receptors: Implications for Cardiovascular Disease and Addiction.Biomolecules. 2025 Jun 11;15(6):855. doi: 10.3390/biom15060855. Biomolecules. 2025. PMID: 40563495 Free PMC article.
-
Deciphering complexity of GPCR signaling and modulation: implications and perspectives for drug discovery.Clin Sci (Lond). 2025 May 20;139(10):463-77. doi: 10.1042/CS20245182. Clin Sci (Lond). 2025. PMID: 40400289 Free PMC article. Review.
-
The GLP-1R as a model for understanding and exploiting biased agonism in next-generation medicines.J Endocrinol. 2024 Apr 11;261(2):e230226. doi: 10.1530/JOE-23-0226. Print 2024 May 1. J Endocrinol. 2024. PMID: 38451873 Review.
-
Exploring Conformational Transitions in Biased and Balanced Ligand Binding of GLP-1R.Molecules. 2025 Jul 31;30(15):3216. doi: 10.3390/molecules30153216. Molecules. 2025. PMID: 40807391 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

