Eliciting antitumor immunity via therapeutic cancer vaccines
- PMID: 40629076
- PMCID: PMC12311208
- DOI: 10.1038/s41423-025-01316-4
Eliciting antitumor immunity via therapeutic cancer vaccines
Abstract
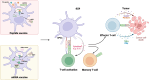
Therapeutic cancer vaccines aim to expand and activate antigen-specific T cells for the targeted elimination of cancer cells. While early clinical trials faced challenges due to suboptimal antigen-specific T-cell activation, recent advancements in antigen discovery and vaccine platform engineering have revitalized the field. This review provides a comprehensive overview of key tumor antigens, including tumor-associated antigens, viral oncoprotein antigens, neoantigens, and cryptic antigens, with a focus on their immunogenicity and therapeutic potential. Advances in our understanding of traditional cancer vaccination targets, in conjunction with the timely identification of novel antigen epitopes, have facilitated the strategic selection of vaccination targets. We also discuss the evolution of cancer vaccine platforms-spanning peptide-based formulations to advanced mRNA vectors-emphasizing innovative strategies to optimize antigen delivery efficiency and adjuvant effects. Efficient antigen delivery and adjuvant selection overcome immune tolerance and tumor-induced immunosuppression. Furthermore, we examine recent clinical trial data and emerging combination approaches that integrate cancer vaccines with other immunotherapies to increase efficacy. While significant progress has been made, challenges remain in improving vaccine-induced T-cell responses, overcoming immune suppression, and translating these advances into effective clinical interventions. Addressing these hurdles will be critical for realizing the full potential of cancer vaccines in immunotherapy.
Keywords: Cancer immunotherapy; Neoantigen; Tumor-associated antigen; Vaccine.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that they have no conflicts of interest. Dr. Yang-Xin Fu is an editorial board member of Cellular & Molecular Immunology, but he has not been involved in peer review or decision-making related to the article.
Figures



Similar articles
-
Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation.Genome Med. 2021 Apr 21;13(1):56. doi: 10.1186/s13073-021-00872-4. Genome Med. 2021. PMID: 33879241 Free PMC article.
-
Immunomodulatory nanoparticles activate cytotoxic T cells for enhancement of the effect of cancer immunotherapy.Nanoscale. 2024 Oct 3;16(38):17699-17722. doi: 10.1039/d4nr01780c. Nanoscale. 2024. PMID: 39257225 Review.
-
DNA-based immunotherapy for cancer: In vivo approaches for recalcitrant targets.Mol Ther. 2025 Jun 4;33(6):2719-2739. doi: 10.1016/j.ymthe.2025.04.008. Epub 2025 Apr 9. Mol Ther. 2025. PMID: 40211538 Review.
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
The Efficacy of Neoantigen-Loaded Dendritic Cell Vaccine Immunotherapy in Non-Metastatic Gastric Cancer.Med Sci (Basel). 2025 Jul 11;13(3):90. doi: 10.3390/medsci13030090. Med Sci (Basel). 2025. PMID: 40700119 Free PMC article. Review.
References
-
- Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol: Mechanisms Dis. 2021;16:223–49. - PubMed
-
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002–14. - PubMed
-
- Vesely MD, Zhang T, Chen L. Resistance mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol. 2022;40:45–74. - PubMed
-
- Galassi C, Chan TA, Vitale I, Galluzzi L. The hallmarks of cancer immune evasion. Cancer Cell. 2024;42:1825–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

