CagriSema drives weight loss in rats by reducing energy intake and preserving energy expenditure
- PMID: 40629149
- PMCID: PMC12286864
- DOI: 10.1038/s42255-025-01324-8
CagriSema drives weight loss in rats by reducing energy intake and preserving energy expenditure
Abstract
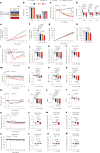
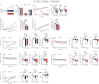
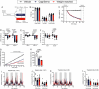
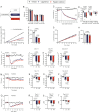
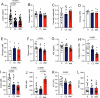
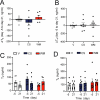
CagriSema is a combination of amylin (cagrilintide) and glucagon-like peptide-1 (semaglutide) analogues being developed for weight management. Here, we show that CagriSema blunts metabolic adaptation in rats. Quantifying CagriSema's action on energy intake and expenditure in rats we observe 12% weight loss with a 39% reduction in food intake. By contrast, pair-feeding causes less-pronounced weight loss, while weight matching requires a 51% decrease in food intake. Therefore, approximately one-third of CagriSema's weight loss efficacy arises from an effect on energy expenditure, the blunting of metabolic adaptation, which contributes to the successful treatment of obesity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.M.J., J.F.H., I.B., J.M.M., B.H., K.P., J.J.F., S.S., A.S., S.L., K.R. and R.E.K. are employed by Novo Nordisk A/S, and some are minor shareholders in Novo Nordisk A/S. The other authors declare no competing interests. All authors declare that this work was not influenced by economic or other types of conflicting interest.
Figures




References
-
- Leibel, R. L., Rosenbaum, M. & Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med.332, 621–628 (1995). - PubMed
-
- Müller, M. J. & Bosy-Westphal, A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring)21, 218–228 (2013). - PubMed
-
- Keys, A., Brožek, J., Henschel, A., Mickelsen, O. & Taylor, H. L. The Biology of Human Starvation Vols 1 and 2 (Univ. Minnesota Press, 1951).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

