Lactoferrin-osteopontin complexes: insights into intestinal organoid bioavailability and gut microbiota modulation
- PMID: 40629168
- PMCID: PMC12238561
- DOI: 10.1038/s42003-025-08460-7
Lactoferrin-osteopontin complexes: insights into intestinal organoid bioavailability and gut microbiota modulation
Abstract
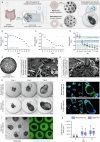
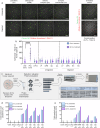
Lactoferrin (LF) and osteopontin (OPN) are key bioactive milk proteins with significant immune-modulatory, gut, and systemic health benefits. We investigated the bioavailability and intestinal uptake dynamics of bovine LF-OPN soluble complexes (SC) and complex coacervates (CC) using a microarrayed high-throughput 3D apical-out intestinal organoid platform, which closely mimics the human intestinal epithelium. Our findings revealed that both SC and CC complexes exhibited cellular uptake compared to individual LF and OPN components. Nevertheless, complexation did not compromise intestinal organoid viability, even following extended exposure. Further, an ex vivo bioreactor-based colon fermentation study using infant fecal microbiota demonstrated that LF-OPN complexes significantly influenced microbial metabolic activities. This modulation leads to enhanced production of short-chain fatty acids (SCFAs), particularly elevating butyrate levels, a key metabolite for sustaining gut health. The phylogenetic analysis highlighted significant shifts in microbial composition, favoring beneficial bacterial families such as Bacteroides fragilis, Phocaeicola dorei, Parabacteroides spp., and Clostridium symbiosum for complex bioactives. Our findings indicate that LF-OPN complexes have significant potential to further optimize infant nutrition by enhancing the bioavailability of bioactive compounds and promoting gut health through microbiota modulation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: L.D., M.E., M.R-C.K., J.O’R., D.G., and O.M. are or were employees of the Société des Produits Nestlé S.A., Switzerland. C.C., J.D., P.S., A.R., and N.B. are or were employees of DOPPL S.A., Switzerland. P.V.d.A. and A.B. are employees of Cryptobiotix S.A., Belgium.
Figures

Similar articles
-
Osteopontin protects from ovalbumin-induced asthma by preserving the microbiome and the intestinal barrier function.mSystems. 2025 Jun 17;10(6):e0038925. doi: 10.1128/msystems.00389-25. Epub 2025 May 22. mSystems. 2025. PMID: 40401951 Free PMC article.
-
Gut Microbiota-Targeted Therapeutics for Metabolic Disorders: Mechanistic Insights into the Synergy of Probiotic-Fermented Herbal Bioactives.Int J Mol Sci. 2025 Jun 7;26(12):5486. doi: 10.3390/ijms26125486. Int J Mol Sci. 2025. PMID: 40564947 Free PMC article. Review.
-
Fecal Microbiota Transplantation Alleviates Airway Inflammation in Asthmatic Rats by Increasing the Level of Short-Chain Fatty Acids in the Intestine.Inflammation. 2025 Jun;48(3):1538-1552. doi: 10.1007/s10753-024-02233-w. Epub 2025 Jan 7. Inflammation. 2025. PMID: 39775370 Free PMC article.
-
The interplay of gut microbiota and intestinal motility in gastrointestinal function.J Smooth Muscle Res. 2025;61:51-58. doi: 10.1540/jsmr.61.51. J Smooth Muscle Res. 2025. PMID: 40619214 Free PMC article. Review.
-
Cannabidiol can affect morphology, morphometry, enzymatic and microbial activity of rabbit digestive system.J Anim Sci. 2024 Jan 3;102:skae376. doi: 10.1093/jas/skae376. J Anim Sci. 2024. PMID: 39661328
References
-
- Lönnerdal, B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr.99, 712S–717S (2014). - PubMed
-
- Lönnerdal, B. Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J. Pediatr.173, S4–S9 (2016). - PubMed
-
- de Weerth, C. et al. Human milk: From complex tailored nutrition to bioactive impact on child cognition and behavior. Crit. Rev. Food Sci. Nutr.63, 7945–7982 (2023). - PubMed
-
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr.173, S10–S15 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

